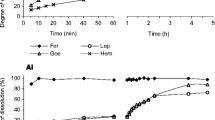
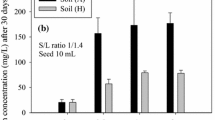
Abstract
A new iron oxide dissolution method designed to measure the abundance of “free” Fe oxide phases and associated elements in soils and sediments has been tested. The method employs a ternary complex of Ti(III), citrate, and ethylenediaminetetraacetate (EDTA) as a reductant and bicarbonate as a proton acceptor. The Ti(III)-citrate-EDTA-HCO3 method dissolved more synthetic amorphous ferric oxide and goethite, but less synthetic hematite, than the dithionite-citrate-HCO3 method of Mehra and Jackson. The production of acidity by the dissolution indicated that Ti(IV) is hydrolyzed to TiO2 during the extractions. The heated dithionite method dissolved 3–6 times more Al from kaolinite and nontronite standard clays than room temperature dithionite, and 4–6 times more Al than the Ti(III)-citrate-EDTA-HCO3 method. Furthermore, the release of Fe from the clay mineral samples consistently and rapidly reached a plateau during multiple extractions by the Ti(III)-citrate-EDTA-HCO3 method, indicating that a well-defined Fe oxide fraction was removed. Fe released by the dithionite method continued to increase with each extraction, suggesting that some release of structural Fe occurred. Tests on two natural sediments and one heavy mineral fraction from the Miocene Cohansey Sand in the New Jersey Coastal Plain suggested that the Ti(III)-citrate-EDTA-HCO3 method removed Fe oxides more effectively and more selectively than the dithionite method. The selectivity of the Ti(III)-citrate-EDTA-HCO3 method is enhanced by rapid extractions at room temperature and low free ligand concentrations.
Similar content being viewed by others
References
Ajmone Marsan, F. and Torrent, J. (1989) Fragipan bonding by silica and Fe oxides in a soil from northwestern Italy: Soil Sci. Soc. Amer. J. 53, 1140–1145.
Atkinson, R. J., Posner, A. M., and Quirk, J. P. (1968) Crystal nucleation in Fe(III) solutions and hydroxide gels: J. Inorg. Nucl. Chem. 30, 2371–2381.
Borggaard, O. K. (1979) Selective extraction of amorphous Fe oxides by EDTA from a Danish sandy loam: J. Soil Sci. 30, 727–734.
Chester, R. and Hughes, M. J. (1967) A chemical technique for the separation of ferro-manganese minerals, carbonate minerals and adsorbed trace elements from pelagic sediments: Chem. Geol. 2, 249–262.
Ericsson, T., Linares, J., and Lotse, E. (1984) A Mossbauer study of the effect of dithionite-citrate-bicarbonate treatment on a vermiculite, a smectite, and a soil: Clay Miner. 19, 85–91.
Fujiwara, S., Nagashima, K., and Codell, M. (1964) Mixed chelate compounds of titanium(III) as studied by electron paramagnetic resonance and spectroscopy: Bull. Chem. Soc. Japan 37, 773–779.
Furrer, G. and Stumm, W. (1986) The coordination chemistry of weathering: I. Dissolution kinetics of α-Al2O3 and BeO: Geochim. Cosmochim. Acta 50, 1847–1860.
Harris, W. G., Carlisle, V. W., and Chesser, S. L. (1987) Clay mineralogy as related to morphology of Florida soils with sandy epipedons: Soil Sci. Soc. Amer. J. 51, 1673–1677.
Heath, G. R. and Dymond, J. (1977) Genesis and transformation of metalliferous sediments from the East Pacific Rise, Bauer Deep and central region northwest Nazca plate: Geol. Soc. Amer. Bull. 88, 723–733.
Holmgren, G. G. S. (1967) A rapid citrate-dithionite ex-tractable iron procedure: Soil Sci. Soc. Amer. Proc. 31, 210–211.
Hudson, R. J. M. and Morel, F.M.M. (1989) Distinguishing between extra- and intracellular Fe in marine phytoplank-ton: Limnol. Oceanogr. 34, 1113–1120.
Jepson, W. B. (1988) Structural iron in kaolinites and in associated ancillary minerals: in Iron in Soils and Clay Minerals, J. W. Stucki, B. A. Goodman, and U. Schwertmann, eds., D. Reidel Publ. Co., 467–536.
Kerr, P. F. (1950) Analytical Data on Reference Clay Material: Amer. Petrol. Inst. Proj. 49, Prelim. Rep. 7, Columbia Univ. Press.
Koehnken, L. and Stallard, R. (1988) Distribution of Fe in clays and coatings in fine-grained sediments from the Orinoco River basin: EOS Trans. Amer. Geophys. Union 69, 1109.
Latimer, W. M. (1956) Oxidation Potentials: 2nd ed., Prentice-Hall, Englewood Cliffs, NJ, 268 pp.
Lim, C. H. and Jackson, M. L. (1982) Dissolution for total analysis: in Methods of Soil Analysis, Part 2, Page, A.L., ed., Amer. Soc. Agron., Madison, WI, 5–7.
Macedo, J. and Bryant, R. B. (1989) Preferential microbial reduction of hematite over goethite in a Brazilian oxisol: Soil Sci. Soc. Amer. J. 53, 1114–1118.
Main, M. S. (1950) Occurrence and Microscopic Examination of Reference Clay Mineral Specimens: Microscopic Examinations: Amer. Petrol. Inst. Proj. 49, Prelim. Rep. 5, Columbia Univ. Press.
McKeague, J. A. and Day, J. H. (1966) Dithionite and oxalate-extractable Fe and Al as aids in differentiating various classes of soils: Can. J. Soil Sci. 46, 13–22.
Mehra, O. P. and Jackson, M. L. (1960) Fe oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate: in Proc. 7th Natl. Conf. on Clays and Clay Minerals, Swineford, A., ed., Pergamon Press, Washington, DC, 317–327.
Mendelovici, E., Yariv, Sh., and Villalba, R. (1979) Fe-bearing kaolinite in Venezuelan laterites: I. Infrared spectroscopy and chemical dissolution evidence: Clay Miner. 14, 323–331.
Poppe, L. J. and Hathaway, J. C. (1979) A metal-membrane mount for x-ray powder diffraction: Clays & Clay Minerals 27, 152–153.
Robbins, J. M., Lyle, M., and Heath, G. R. (1984) A sequential extraction procedure for partitioning elements among co-existing phases in marine sediments: Reference 84-3, College of Oceanography, Oregon State University, Corvallis, OR, 64 pp.
Rozenson, I. and Heller-Kallai, L. (1976) Reduction and oxidation of Fe3 in dioctahedral smectites-1: Reduction with hydrazine and dithionite: Clays & Clay Minerals 24, 271–282.
Rude, P. D. and Aller, R. C. (1989) Early diagenetic alteration of lateritic particle coatings in Amazon continental shelf sediments: J. Sediment Petrol. 59, 704–716.
Ryan, J. N. and Gschwend, P. M. (1990) Colloid mobilization in two Atlantic Coastal Plain aquifers: Field studies: Water Resour. Res. 26, 307–322.
Schulze, D. G. (1984) The influence of aluminum on iron oxides. VIII. Unit-cell dimensions of Al-substituted goethites and estimation of Al from them. Clays & Clay Minerals 32, 36–44.
Schwertmann, U. (1973) Use of oxalate for Fe extraction from soils: Can. J. Soil Sci. 53, 244–246.
Shuman, L. M. (1982) Separating soil Fe- and manganese-oxide fractions for microelement analysis: Soil Sci. Soc. Amer. J. 46, 1099–1102.
Smith, B. R. and Callahan, L. L. (1987) Soils with Bx horizons in the upper coastal plains of South Carolina: Soil Sci. Soc. Amer. J. 51, 158–164.
Stucki, J. W., Golden, D. C, and Roth, C. B. (1984) Effects of reduction and reoxidation of structural iron on the surface charge and dissolution of dioctahedral smectites: Clays & Clay Minerals 32, 350–356.
Tardy, Y. and Nahon, D. (1985) Geochemistry of laterites, stability of Al-goethite, Al-hematite, and Fe3+-kaolinite in bauxites and ferricretes: An approach to the mechanism of concretion formation: Amer. Jour. Sci. 285, 865–903.
Tessier, A., Campbell, P. G. C, and Bisson, M. (1979) Sequential extraction procedure for the speciation of particulate trace metals: Anal. Chem. 51, 844–851.
Torrent, A., Schwertmann, U., and Barron, V. (1987) The reductive dissolution of synthetic goethite and hematite in dithionite: Clay Miner. 22, 329–337.
Westall, J., Zachary, J., and Morel, F. M. M. (1976) MINEQL, a computer program for the calculation of chemical equilibrium composition of aqueous systems: Technical Note 18, Dept. Civil Eng., Massachusetts Inst. Tech., 91 pp.
Zehnder, A. J. B. and Wuhrmann, K. (1976) Titanium(III) citrate as a nontoxic oxidation-reduction buffering system for the culture of obligate anaerobes: Science 194, 1165–1166.
Zinder, B., Furrer, G., and W. Stumm (1986) The coordination chemistry of weathering: II. Dissolution of Fe(III) oxides: Geochim. Cosmochim. Acta 50, 1861–1869.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ryan, J.N., Gschwend, P.M. Extraction of Iron Oxides from Sediments Using Reductive Dissolution by Titanium(III). Clays Clay Miner. 39, 509–518 (1991). https://doi.org/10.1346/CCMN.1991.0390506
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1991.0390506