Abstract
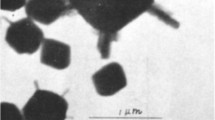
Transmission electron microscopic (TEM) examination has shown that multi-domainic crystals of synthetic goethite consist of almost parallel intergrowths, each of which is slightly misoriented with respect to its neighbors. These intergrowths emanate from a central nucleus within the crystal. They can nucleate along both the x and y crystal axes, but subsequent growth is mainly in the z direction.
The formation of multi-domainic goethites from ferrihydrite was favored by high pH (≥ 13) and, at lower pHs, by the addition of NaNO3 to the system. Decreasing the temperature of synthesis from 70° to 20°C also enhanced domain formation. The nucléation of domains was confined to the initial stage of goethite formation. Domains probably formed when crystal growth was very rapid or when adsorbed species blocked the appropriate sites on the nucleus material.
Similar content being viewed by others
References
Atkinson, R. J., Posner, A. M., and Quirk, J. P. (1968) Crystal nucléation in Fe(III) solutions and hydroxide gels: J. Inorg. Nucl. Chem. 30, 2371–2381.
Buerger, M. J. (1934) The lineage structure of crystals: Z. Krist. 89, 195–220.
Cornell, R. M. and Giovanoli, R. (1985) Effect of solution conditions on the proportion and morphology of goethite formed from ferrihydrite: Clays & Clay Minerals 33, 424–432.
Cornell, R. M., Mann, S., and Skamulis, A. J. (1983) A high-resolution electron microscopy examination of domain boundaries in crystals of synthetic goethite; J. Chem. Soc. Faraday Trans. 1 79, 2679–2684.
Cornell, R. M., Posner, A. M., and Quirk, J. P. (1974) Crystal morphology and dissolution of goethite: J. Inorg. Nucl. Chem. 36, 1937–1946.
Davis, J. A. and Leckie, J. O. (1978) Surface ionization and complexation at the oxide/water interface. II. Surface properties of amorphous iron oxyhydroxide and adsorption of metal ions: J. Colloid Interface Sci. 67, 90–107.
Lewis, D. G. and Schwertmann, U. (1980) The effect of [OH-] on the goethite produced from ferrihydrite under alkaline conditions: J. Colloid Interface Sci. 78, 543–553.
Mann, S., Cornell, R. M., and Schwertmann, U. (1985) The influence of aluminium on iron oxides: a high-resolution electron microscopy study of aluminous goethites: Clay Miner. 20, 255–262.
Read, W. T., Jr. and Shockley, W. (1952) Dislocation models of grain boundaries: in Imperfections in Nearly Perfect Crystals, W. Shockley, J. H. Holloman, R. Mauer, and F. Seitz, eds., Wiley, New York, 352–376.
Schulze, D. G. and Schwertmann, U. (1984) The influence of aluminium on iron oxides. X. The properties of Al-substituted goethites: Clay Miner. 19, 521–529.
Schwertmann, U. (1984) The influence of aluminium on iron oxides. IX. Dissolution of Al-goethites in 6 M HCl. Clay Miner. 19, 9–19.
Schwertmann, U. and Murad, E. (1983) The effect of pH on the formation ofgoethite and hematite from ferrihydrite: Clays & Clay Minerals 31, 277–284.
Sidhu, P. S., Gilkes, R. J., Cornell, R. M., Posner, A. M., and Quirk, J. P. (1981) Dissolution of iron oxides and oxy-hydroxides in hydrochloric and perchloric acids: Clays & Clay Minerals 29, 269–276.
Smith, K. L. and Eggleton, R. A. (1983) Botryoidal goethite: a transmission electron microscope study: Clays & Clay Minerals 31, 392–396.
Watari, F., Delavignette, P., and Amelinckx, S. (1979) Electron microscopic study of dehydration transformations. II. The formation of “superstructures” on the dehydration of goethite and diaspore: J. Solid State Chem. 29, 417–427.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cornell, R.M., Giovanoli, R. Factors That Govern the Formation of Multi-Domainic Goethites. Clays Clay Miner. 34, 557–564 (1986). https://doi.org/10.1346/CCMN.1986.0340509
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1986.0340509