Abstract
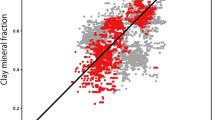
Multivariate statistical analyses of geochemical, mineralogical, and cation-exchange capacity (CEC) data from a Venezuelan oil well were used to construct a model which relates elemental concentrations to mineral abundances. An r-mode factor analysis showed that most of the variance could be accounted for by four independent factors and that these factors were related to individual mineral components: kaolinite, illite, K-feldspar, and heavy minerals. Concentrations of Al, Fe, and K in core samples were used to estimate the abundances of kaolinite, illite, K-feldspar, and, by subtraction from unity, quartz. Concentrations of these elements were also measured remotely in the well by geochemical logging tools and were used to estimate these mineral abundances on a continuous basis as a function of depth. The CEC was estimated from a linear combination of the derived kaolinite and illite abundances. The formation’s thermal neutron capture cross section estimated from the log-derived mineralogy and a porosity log agreed well with the measured data. Concentrations of V, among other trace elements, were modeled as linear combinations of the clay mineral abundances. The measured core V agreed with the derived values in shales and water-bearing sands, but exceeded the clay-derived values in samples containing heavy oil. The excess V was used to estimate the V content and API Gravity of the oil. The log-derived clay mineralogy was used to help distinguish nonmarine from transitional depositional environments. Kaolinite was the dominant clay in nonmarine deposits, whereas transitional sediments contained more illite.
Similar content being viewed by others
References
Adams, J. A. S., Osmond, J. K., and Rogers, J. J. W. (1959) The geochemistry of thorium and uranium: Phys. Chem. Earth 3, 298–348.
Adams, J. A. S. and Weaver, C. E. (1958) Thorium-to-uranium ratios as indicators of sedimentary processes: example of concept of geochemical facies: Bull. Amer. Assoc. Pet. Geol. 42, 387–430.
Brindley, G. W., Kao, C-C., Harrison, J. L., Lipsicas, M., and Raythatha, R. (1986) The relation between structural disorder and other characteristics of kaolinites and dickites: Clays & Clay Minerals 34 (in press).
Cody, R. D. (1971) Adsorption and the reliability of trace elements as environmental indicators for shales: J. Sediment. Petrol. 41, 461–471.
Davis, J. C. (1973) Statistics and Data Analysis in Geology: Wiley, New York, 473–533.
Decarreau, A. (1985) Partitioning of divalent transition elements between octahedral sheets of trioctahedral smectites and water: Geochim. Cosmochim. Acta 49, 1537–1544.
Everett, R., Herron, M., and Pirie, G. (1983) Log responses and core evaluation case study technique: field and laboratory procedures: Soc. Prof. Well Log Analysts Ann. Meet., Calgary, Alberta, 1983, Paper OO.
Gold, C. M., Cavell, P. A., and Smith, D. G. W. (1983) Clay minerals in mixtures: sample preparation, analysis, and statistical interpretation: Clays & Clay Minerals 31, 191–199.
Grim, R. E. (1968) Clay Mineralogy: McGraw-Hill, New York, 185–233.
Hinckley, D. N. (1963) Variability in “crystallinity” values among the kaolin deposits of the coastal plain of Georgia and South Carolina: in Clays and Clay Minerals, Proc. 11th Natl. Conf., Ottawa, Ontario, 1963, W. F. Bradley, ed., Pergamon Press, New York, 229–235.
Hodgson, M. and Dudeney, A. W. L. (1984) Estimation of clay proportions in mixtures by X-ray diffraction and computerized chemical mass balance: Clays & Clay Minerals 32, 19–28.
Imbrie, J. and Poldervaart, A. (1959) Mineral compositions calculated from chemical analyses of sedimentary rocks: J. Sediment. Petrol. 29, 588–595.
Kapo, G. (1978) Vanadium: key to Venezuelan fossil hydrocarbons: in Bitumen, Asphalts, and Tar Sands, G. V. Chilingarian and T. F. Yen, eds., Elsevier, Amsterdam, 213–241.
Lonnie, T. P. (1982) Mineralogic and chemical comparison of marine, nonmarine, and transitional clay beds on south shore of Long Island, New York: J. Sediment. Petrol. 52, 529–536.
Mestdagh, M. M., Vielvoye, L., and Herbillon, A. J. (1980) Iron in kaolinite: II. The relationship between kaolinite crystallinity and iron content: Clay Miner. 15, 1–13.
Miesch, A. T. (1962) Computing mineral compositions of sedimentary rocks from chemical analyses: J. Sediment. Petrol. 32, 217–225.
Murali, A. V., Parthasarathy, R., Mahadevan, T. M., and SankarDas, M. (1983) Trace element characteristics, REE patterns and partition coefficients of zircons from different geological environments—a case study on Indian zircons: Geochim. Cosmochim. Acta 47, 2047–2052.
Parham, W. E. (1966) Lateral variations of clay mineral assemblages in modern and ancient sediments: in Proc. Int. Clay Conf., Jerusalem, 1966, Vol. 1, L. Heller and A. Weiss, eds., Israel Prog. Sci. Transi., Jerusalem, 135–145.
Pearson, M. J. (1978) Quantitative clay mineralogical analyses from the bulk chemistry of sedimentary rocks: Clays & Clay Minerals 26, 423–433.
Pirie, G. and Everett, R. (1984) Depositional environments and invasion profiling in heavy oil sands, eastern Venezuela: a case study: Amer. Assoc. Petrol. Geol. Bull. 68, 516 (abstract).
Potter, P. E., Maynard, J. B., and Pryor, W. A. (1980) Sed-imentology of Shale: Springer-Verlag, New York, 303 pp.
Rankama, K. and Sahama, T. G. (1950) Geochemistry: University of Chicago Press, Chicago, 557–569.
Ridge, M. J. (1983) A combustion method for measuring the cation exchange capacity of clay materials: Log Analyst 24, 6–11.
Tardy, Y. (1975) Element partition ratios in some sedimentary environments: Sci. Geol. Bull. 28, 59–73.
Turekian, K. K. and Wedepohl, K. H. (1961) Distribution of the elements in some major units of the earth’s crust: Geol. Soc. Amer. Bull. 72, 175–192.
Waxman, M. H. and Smits, L. J. M. (1968) Electrical conductivities in oil-bearing shaly sands: Soc. Prof. Eng. J. 243, 107–122.
Weaver, C. E. and Pollard, L. D. (1975) The Chemistry of Clay Minerals: Elsevier, Amsterdam, 205 pp.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Herron, M.M. Mineralogy from Geochemical Well Logging. Clays Clay Miner. 34, 204–213 (1986). https://doi.org/10.1346/CCMN.1986.0340211
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1986.0340211