Abstract
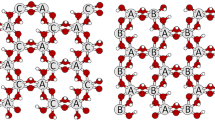
The synthesis of 10-Å hydrated kaolinite was accomplished by: (1) direct reaction of HF with a dimethyl sulfoxide (DMSO)-kaolinite intercalate and water washing; (2) methanol washing of a DMSO-kaolinite intercalate followed by reaction with any alkali fluoride salt and water washing; and (3) room-temperature (RT) water-washing of a methanol-washed DMSO-kaolinite intercalate. In all syntheses the optimum yield required a kaolinite in which DMSO was bound strongly to the interlayer surface. In the first synthesis, water inclusion between clay layers appeared to be facilitated by the reduction of cohesive interlayer forces brought about by replacement of surface and edge OH− by F−. The fluorination reaction was accomplished either by direct reaction of HF or by HF produced through the hydrolysis of NH4F at 60°C. In the second synthesis, intercalated DMSO was replaced by methanol. F− solvated readily in methanol but not in DMSO. Consequently, F− produced through hydrolysis of the alkali fluoride salt entered the interlayer space and contributed to the fluorination reaction. Furthermore, the diffusion of methanol out of the interlayer space during the RT-washing step was slowed by F≈ solvation which aided the exchange of methanol for water. High yields of 10-Å kaolinite hydrate were obtained irrespective of choice of alkali fluoride salt. The third synthesis was dependent on matching the diffusion of methanol out of the interlayer space with diffusion of water into this space. At room temperature the diffusion rates were close enough to maintain the clay in the expanded state throughout the hydration process, and high yields of 10-Å kaolinite hydrate were obtained. At 60°C the diffusion rates were too dissimilar, and very low yields of hydrate were obtained.
Similar content being viewed by others
References
Barrios, J., Plancon, A., Cruz, M. I., and Tchoubar, C. (1977) Qualitative and quantitative study of stacking faults in a hydrazine treated kaolinite-relationship with the infrared spectra: Clays & Clay Minerals 25, 422–429.
Costanzo, P. M., Clemency, C.V., and Giese, R. F., Jr. (1980) Low temperature synthesis of a 10-Å hydrate of kaolinite using dimethyl sulfoxide and ammonium fluoride: Clays & Clay Minerals 28, 155–156.
Costanzo, P. M., Giese, R. F., Jr., Lipsicas, M., and Straley, C. (1982) Synthesis of a quasi-stable kaolinite and heat capacity of interlayer water: Nature 296, 549–551.
Costanzo, P. M., Giese, R. F., Jr., and Clemency, C. V. (1984a) Synthesis of a 10-Å hydrated kaolinite: Clays & Clay Minerals 32, 29–35.
Costanzo, P. M., Giese, R. F., Jr., and Lipsicas, M. (1984b) Static and dynamic structure of water in hydrated kaolin-ites: I. The static structure: Clays & Clay Minerals 32, 419–428.
Martin, D. (1975) Solvation and association in DMSO: in Dimethyl Sulfoxide, D. Martin, H. G. Hauthal, and E. S. Halberstadt, eds., Van Nostrand-Reinhold, Wokingham, United Kingdom, 500 pp.
Olejnik, S., Aylmore, L. A. G., Posner, A. M., and Quirk, J. P. (1968) Infrared spectra of kaolin mineral-dimethyl sul-phoxide complexes: J. Phys. Chem. 72, 241–249.
Wolfe, R. and Giese, R. F., Jr. (1978) The stability of fluorine analogs of kaolinite: Clays & Clay Minerals 26, 76–78.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Raythatha, R., Lipsicas, M. Mechanism of Synthesis of 10-Å Hydrated Kaolinite. Clays Clay Miner. 33, 333–339 (1985). https://doi.org/10.1346/CCMN.1985.0330409
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1985.0330409