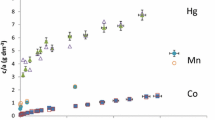
Abstract
Ion-exchange experiments in expanding clay minerals conducted over a wide range of surface ionic compositions and ionic strength produce variable mass-action selectivity coefficients. When the exchanging ions are of unequal charge, tactoid structure appears to influence selectivity, although configurational entropy of adsorbed ions may also generate variable selectivity. The degree of deviation from ideal mass-action exchange is related to the dissimilarity of the ions undergoing exchange. Data involving trivalent ion adsorption on smectites suggest that mass-action is a poor approximation when the adsorbing and desorbing ions have different hydration energies and charge. No form of exchange equation is successful in describing ion exchange for a wide range of experimental conditions, although the fluctuation of the selectivity coefficient follows consistent trends with changing experimental conditions. The strong adsorption of high-charge ions on clays is not exothermic, but must be driven by the increasing disorder of ions and/or water.
Резюме
Показано, что ионно-обменные эксперименты, проведенные на расширяющихся глинистых минералах в пределах поверхностных ионных составов и ионных сил дают переменные коэффициенты масс-действенной селективности. Когда обменные ионы имеют неравный заряд, тактоидная структура, по видимому, влияет на селективность, хотя конфигурационная энтропия адсорбированных ионов тоже может вызывать переменную селективность. Степень отклонения от идеального масс-действенного обмена связана с различием ионов, вовлеченных в обмен. Данные, включающие трехвалентную адсорбцию ионов смектитами, указывают на то, что масс-действие является плохим приближением, когда адсорбирующие и десорбирующие ионы имеют разные энергии гидратации и заряд. Ни одна форма уравнения обмена не является подходящей для описания ионного обмена для широкого диапазона экспериментальных условий, хотя колебание коэффициента селективности следует последоветельным тенденциям с изменением экспериментальных условий. Сильная адсорбция глинами ионов с высокими зарядами не является экзотермической, а должна поддерживаться увеличивающимся разупорядочиванием ионов и/или воды. [N.R.]
Resümee
Experimente zum Ionenaustausch in quellfähigen Tonmineralen, wobei deren Oberflächen eine unterschiedliche Ionenzusammensetzung und Ionenstärke aufwiesen, fähren zu unterschiedlichen Selektivitätskoeffizienten. Wenn die austauschenden Ionen von ungleicher Ladung sind, scheint eine taktoide Struktur die Selektivität zu beeinflussen, obwohl auch die Konfigurationsentropie des adsorbierten Ions eine unterschiedliche Selektivität hervorrufen kann. Der abweichungsgrad vom idealen Austausch nach dem Massenwirkungsgesetz steht in Zusammenhang mit der Unterschiedlichkeit der Ionen, die am Austausch teilnehmen. Ergebnisse bei der Adsorption dreiwertiger Ionen an Smektit deuten darauf hin, daß das Massenwirkungsgesetz eine ungenaue Annäherung ist, wenn das zu adsorbierende und das zu desorbierende Ion unterschiedliche Hydratationsenergien und Ladungen haben. Keine Art von Austauschgleichung kann den Ionenaustausch für einen großen Bereich von experimentellen Bedingungen genau beschreiben, obwohl die Änderung des Selektivitätskoeffizienten bei sich ändernden experimentellen Bedingungen bestimmten Trends folgt. Die starke Adsorption von hochwertigen Ionen an Tonen ist nicht exotherm, sondern muß durch die zunehmende Unordnung der Ionen und/oder des Wassers bewirkt werden. [U.W.]
Résumé
Des expériences d’échange d’ions dans des minéraux argileux en expansion faits sur une large gamme de compositions ioniques et de force ionique de surface produisent des coéficients de sélectivité d’action en masse variables. Lorsque les ions échangeants sont de charge inégale, une structure tactoide semble influencer la sélectivité, quoique l’entropie de configuration des ions adsorbés peut aussi générer une sélectivité variable. Le degré de déviation de l’échange d’action en masse idéal est apparenté à la dissimilar ité des ions subissants l’échange. Des donneés impliquant l’adsorption d’ions trivalents sur des smectites suggère que l’action en masse est une pauvre approximation lorsque les ions adsorbants et désorbants ont des énergies d’hydration et une charge différentes. Aucune forme d’équation d’échange ne réeussit à décrire l’échange d’ions pour une large gamme de conditions expérimentales, quoique la fluctuation du coéficient de sélectivité suit des directions régulières suivant des changements de conditions expérimentales. L’adsorption forte d’ions à haute charge sur les argiles n’est pas exothermique mais doit être pousseé par le désordre croissant d’ions et/ou d’eau. [D.J.]
Similar content being viewed by others
References
Banin, A. (1968) Ion exchange isotherms of montmorillonite and structural factors affecting them: Isr. J. Chem. 6, 27–36.
Barrer, R. M. and Falconer, J. D. (1956) Ion exchange in feld-spathoids as a solid state reaction: Proc. Roy. Soc, London A 236, 227–249.
Coleman, N. J. (1952) A thermochemical approach to the study of ion exchange: Soil Sci. 74, 115–125.
Davis, L. E. (1950) Ionic exchange and statistical thermodynamics: I. Equilibria in simple exchange systems: J. Colloid Sci. 5, 71–79.
Eriksson, E. (1952) Cation-exchange equilibria on clay minerals: Soil Sci. 74, 103–113.
Fink, D. H., Nakayama, F. S., and McNeal, B. L. (1971) Demixing of exchangeable cations in free-swelling bentonite clay: Soil Sci. Soc. Amer. Proc. 35, 552–555.
Frysinger, G. R. and Thomas, H. C. (1960) Adsorption studies on clay minerals VII. Yttrium-cesium and cerium(IH)-cesium on montmorillonite: J. Phys. Chem. 64, 224–228.
Gaines, G. L. and Thomas, H. C. (1953) Adsorption studies on clay minerals. II. A formulation of the thermodynamics of exchange adsorption: J. Chem. Phys. 21, 714–718.
Gaines, G. L. and Thomas, H. C. (1955) Adsorption studies on clay minerals. V. Montmorillonite-cesium-strontium at several temperatures: J. Chem. Phys. 23, 2322–2326.
Garcia-Miragaya, J. and Page, A. L. (1977) Influence of exchangeable cation on the sorption of trace amounts of cadmium by montmorillonite: Soil Sci. Soc. Amer. J. 41, 718–721.
Gilbert, M. and van Bladel, R. (1970) Thermodynamics and thermochemistry of the exchange reaction between NH4+ and Mn2+ in a montmorillonite clay: J. Soil Sci. 21, 38–49.
Harvey, J. F., Redfern, J. P., and Salmon, J. E. (1966) Selectivity in uni-bivalent ion exchange reactions: Trans. Faraday Soc. 62, 198–203.
Hutcheon, A. T. (1966) Thermodynamics of cation exchange on clay: Ca-K-montmorillonite: J. Soil Sci. 17, 339–355.
Keren, R. (1979) The effect of hydroxyl-aluminum precipitation on the exchange properties of montmorillonite: Clays & Clay Minerals 27, 303–304.
Krishnamoorthy, C. and Overstreet, R. (1949) Theory of ion-exchange relationships: Soil Sci. 68, 307–315.
Krishnamoorthy, C. and Overstreet, R. (1950) An experimental evaluation of ion-exchange relationships: Soil Sci. 69, 41–53.
Laudelout, H., van Bladel, R., Bolt, G. H., and Page, A. L. (1968) Thermodynamics of heterovalent cation exchange reactions in a montmorillonite clay: Trans. Faraday Soc. 64, 1477–1488.
Maes, A., Peigneur, P., and Cremers, A. (1976) Thermodynamics of transition metal ion exchange in montmorillonite: in Proc. Int. Clay Conf., Mexico City, 1975, S. W. Bailey, ed., Applied Publishing Ltd., Wilmette, Illinois, 319–329.
Marshall, C. E. (1964) The Physical Chemistry and Mineralogy of Soils: John Wiley, New York, 388 pp.
McBride, M. B. (1976) Exchange and hydration properties of Cu2+ on mixed-ion Na+-Cu2+ smectites: Soil Sci. Soc. Amer. J. 40, 452–456.
McBride, M. B. (1979) An interpretation of cation selectivity variations in M+-M+ exchange on clays: Clays & Clay Minerals 27, 417–422.
McBride, M. B. and Bloom, P. R. (1977) Adsorption of aluminum by a smectite II. An Al3+-Ca2+ exchange model: Soil Sci. Soc. Amer. J. 41, 1073–1077.
Nye, P., Craig, D., Coleman, N. T., and Ragland, J. L. (1961) Ion exchange equilibria involving aluminum: Soil Sci. Soc. Amer. Proc. 25, 14–17.
Peigneur, P., Maes, A., and Cremers, A. (1975) Heterogeneity of charge density distribution in montmorillonite as inferred from cobalt adsorption: Clays & Clay Minerals 23, 71–75.
Shainberg, I. and Kemper, W. D. (1966) Electrostatic forces between clay and cations calculated and inferred from electrical conductivity: Clays & Clay Minerals 14, 117–132.
Shainberg, I. and Otoh, H. (1968) Size and shape of montmorillonite particles saturated with Na/Ca ions (inferred from viscosity and optical measurements): Isr. J. Chem. 6, 251–259.
Sherry, H. S. (1969) The ion exchange of zeolites: in Ion Exchange and Solvent Extraction, vol. 2, J. A. Marinsky, ed., Marcel Dekker, New York, 89–131.
Thomas, H. C. (1965) Toward a connection between ionic equilibrium and ionic migration in clay gels: in Plant Nutrient Supply and Movement, Tech. Rep. Series No. 48, Int. Atomic Energy Agency, Vienna, 4–19.
Author information
Authors and Affiliations
Additional information
Agronomy Paper No 1335.
Rights and permissions
About this article
Cite this article
McBride, M.B. Interpretation of the Variability of Selectivity Coefficients for Exchange Between Ions of Unequal Charge on Smectites. Clays Clay Miner. 28, 255–261 (1980). https://doi.org/10.1346/CCMN.1980.0280402
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1980.0280402