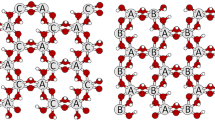
Abstract
Kaolinites of all kinds (fine, ‘fireclay,’ ‘type IV,’ etc.), some of which do not expand or expand incompletely with the usual intercalation methods used for comparison, are expanded completely by treatment of dry (110°C) clay with dry CsCl salt, followed by contact with hydrazine for 1 day at 65°C and then with DMSO overnight at 90°C. Comparison treatments were grinding in KOAc, soaking in hydrazine, and Li-DMSO, as well as combination of these. Following the Cs-hydrazine-DMSO treatment, the 7.2 Å spacing of 1:1 dioctahedral layer silicates shifts to 11.2 Å and the 11.2 Å/(7.2 + 11.2 Å) ratio ≃1.0. The trioctahedral 1:1 layer silicates and chlorite are not expanded by the Cs-hydrazine-DMSO procedure.
Резюме
Каолиниты всех типов /мелкозернистый, огнеупорная глина,“тип” и т.д./, некоторые из которых не расширяются или расширяются неполностью при обычных методах интеркалации, использовавшихся для сравнения,были расширены полностью путем обработки сухой /110°C/ глины сухой солью CsCl, после чего следовал контакт с гидрозином в течение 1 дня при 65°C и затем с диметил-сульфоксидом в течение ночи при 90°C. Для сравнения, обработки проводились ацетатом калия, пропиткой гидрозином и Li-диметилсульфоксидом,а также их смесями. После Cs-гидрозин-диметилсульфоксидной обработки промежуток в 7,2Å между силикатными диоктаэдрическими слоями 1:1 расширился до 11,2Å и отношения 11,2Å/ (7,2+11,2Å)=1,0. Силикаты с триоктаэдрическими слоями 1:1 и хлорит не расширились в результате Cs-гидрозин-диметилсульфоксидной обработки.
Kurzreferat
Kaoliniten aller Art (fein,feuerfest,Typ IV usw.) von denen ei nige sich nicht oder nur unvollständig, mit den gewöhnlichen, zum Vergleich benutzten Einlagerungsmethoden ausdehnen, wurden vollständig geschwollen, durch Behandlung des trockenen(110°C) Tons mit trocknem CsCl Salz, und nach folgend mit Hydrazin für einen Tag bei 65°C und dann mit DMSO über Nacht bei 90°C. Verglelchsbehandlungen waren: Vermahlen in KOAc, Einweichen in Hydrazin und Li-DMSO, sowohl wie eine Kombination dieser Methoden. Nach der Cs-Hydrazin-DMSO Behandlung, ändert sich der 7,2A Zwischenraum der 1 /1 Diokta hedrischen Schichtsilikate auf 11,2A und das Verhäitnis 11,2A (7,2+11,2A) ≂ 1,0. Die trioktahedrischen 1 / 1 Schichtsilikate und Chlorite schwellen nicht mit der Cs-Hydrazin-DMSO Methode.
Similar content being viewed by others
References
Abdel-Kader, F. H., Jackson, M. L. and Lee, G. B. (1976) Soil ka-olinite, vermiculite and chlorite identification by an improved lithium DMSO X-ray diffraction test: Agron. Abstr. p. 194; J. Soil Sci. Soc. Am. (in press, 1977).
Andrew, R. W., Jackson, M. L. and Wada, K. (1960) Intersalation as a technique for differentiation of kaolinite from chloritjc minerals by X-ray diffraction: Soil Sci. Soc. Am. Proc. 24, 422–424.
Bailey, S. W. and Langston, R. B. (1969) Anauxite and kaolinite structures identical: Clays & Clay Minerals 17, 241–243.
Cruz, M., Jacobs, H. and Fripiat, J. J. (1972) The nature of the inter-layer bonding in kaolin minerals: Proc. Int. Clay Conf., Madrid, pp. 35–44.
Farmer, V. C. and (in part) Russell, J. D. (1966) Effect of particle size and structure on the vibrational frequencies of layer silicates: Spec-trochim. Acta 22, 389–398.
Giese, R. F. (1973) Interlayer bonding in kaolinite, dickite and nacrite: Clays & Clay Minerals 21, 145–149.
Gieseking, J. E. (1939) Mechanism of cation exchange in montmorillonite-beidellite-nontronite type of clay minerals: Soil Sci. 47, 1–13.
Herbillon, A. J., Mestdagh, M. M., Vielvoye, L. and Derouane, E. G. (1976) Iron in kaolinite with special reference to kaolinite from tropical soils: Clay Miner. 11, 201–219.
Jackson, M. L. (1974) Soil Chemical Analysis—Advanced course, 2nd edition, 9th printing, 895 pp. Published by the author, Madison.
Jackson, M. L. and Hellman, N. N. (1941) X-ray diffraction procedure for positive differentiation of montmorillonite from hydrous mica: Soil Sci. Soc. Am. Proc. 6, 133–145.
Lee, S. Y., Jackson, M. L. and Brown, J. L. (1975) Micaceous occlusions in kaolinite observed by ultramicrotomy and high resolution electron microscopy: Clays & Clay Minerals 23, 125–129.
Miller, J. G. and Oulton, T. D. (1970) Prototropy in kaolinite during percussive grinding: Clays & Clay Minerals 18, 313–323.
Olejnik, S., Aylmore, L. A. G., Posner, A. M. and Quirk, J. P. (1968) Infrared spectra of kaolin-mineral-dimethyl sulfoxide complexes: J. Phys. Chem. 72, 241–249.
Olejnik, S., Posner, A. M. and Quirk, J. P. (1970) The intercalation of polar organic compound into kaolinite: Clay Miner. 8, 421–434.
Ormsby, W. C., Shartsis, J. M. and Woodside, K. H. (1962) Exchange behavior of kaolins of varying degree of crystallinity: J. Am. Ceram. Soc. 45, 361–366.
Pauling, L. (1970) General Chemistry, 3rd edition: W. H. Freeman, San Francisco.
Range, K. J., Range, A. and Weiss, A. (1969) Fire-clay type kaolinite or fire-clay mineral? Experimental classification of kaolinite-halloy-site minerals: Proc. 3rd Int. Clay Conf., Tokyo, Vol. 1, pp. 3–13.
Theng, B. K. G. (1974) The Chemistry of Clay-Organic Reactions: John Wiley, New York.
Thiry, M. and Weber, F. (1977) Convergence de compartement entre les interstratifies kaolinite-smectite et les fireclays: Clay Miner. 12, 83–91.
Wada, K. (1961) Lattice expansion of kaolin minerals by potassium acetate treatment: Am. Mineral. 46, 78–91.
Wada, K. and Yamada, H. (1968) Hydrazine intercalation-intersalation for differentiation of kaolin minerals from chlorites: Am. Mineral. 53, 334–339.
Weiss, A., Thielepape, W., Göring, G., Ritter, W. and Schäfer, H. (1963) Kaolinite-Einlagerungs-Verbindungen: Proc. 1st Int. Clay Conf, Stockholm, Vol. 1, pp. 287–305.
Weiss, A., Thielepape, W. and Orth, H. (1966) Neue Kaolinite-Ein-lagerungsverbindungen: Proc. 2nd Int. Clay Conf., Jerusalem, Vol. 1, pp. 277–293.
Weiss, A., Becker, H. O., Orth, H., Mai, G. and Wimmer, M. (1975) Mechanism of the intercalation into kaolinite and related silicates: Proc. 4th Int. Clay Conf., Mexico City, p. 411.
Wiewiora, A. and Brindley, G. W. (1969) Potassium acetate intercalation in kaolinite and its removal: effect of material characteristics: Proc. 3rd Int. Clay Conf, Tokyo, Vol. 1, pp. 723–733.
Yariv, S. (1975) Infra-red study of interaction between caesium chloride and kaolinite: J. Chem. Soc. Faraday Trans. I. 75, 674–684.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jackson, M.L., Abdel-Kader, F.H. Kaolinite Intercalation Procedure for all Sizes and Types with X-Ray Diffraction Spacing Distinctive from other Phyllosilicates. Clays Clay Miner. 26, 81–87 (1978). https://doi.org/10.1346/CCMN.1978.0260201
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1978.0260201