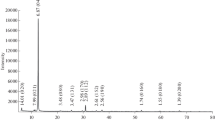
Abstract
A hydromuscovite in association with gypsum and anhydrite was collected from the Shakanai mine, Akita Prefecture, Japan. Chemical composition: SiO2 47·14%; TiO2 0·34%; A12O3 37·09%; Fe2O3 0·49%; MgO 0·83%; CaO 0·57%; Na2O 0·35%; K2O 7·10%; H2O+ 5.18%; H20–0·99%; P2O5 0·01%; total 100·09%. Differential thermal and i.r. absorption analyses were similar to those of hydromuscovite. The X-ray diffraction pattern differed clearly from those of the 1M and/or 2M1 polymorphs and it was similar to that of the 2M2 polymorph, which is known to occur in lepido-lites.
Résumé
Une hydromuscovite en association avec du gypse et de l’anhydrite a été recueillie de la mine de Shakanai, Préfecture d’Akita, Japon. Composition chimique: SiO2 47,14%; TiO2 0,34%; A12O3 37,09%; Fe2O3 0,49%; MgO 0,83%; CaO 0,57%; Na2O 0,35%; K2O 7,10%; H2O 5,18%; H2O− 0,99%; P2O5 0,01%; total 100,09%. Les analyses différentielles thermiques et d’absorption d’infra-rouge étaient similaires à celles de l’hydromuscovite. Un modèle de diffraction des rayons X différait nettement de ceux des polymorphes 1M et/ou 2M1 et était identique à celui du polymorphe 2M2 qui est connu pour se produire dans les lépidolites.
Zusammenfassung
Ein Hydromuskowit begleitet von Gips und Anhydrit wurde aus den Shakanai Minen, Akita Prefektur, Japan bezogen. Die chemische Zusammensetzung war wie folgt: SiO2 47,14%; TiO, 0,34%; A12O3 37,09%; Fe2O3 0,49%; MgO 0,83%; CaO 0,57%; Na2O 0,35%; K2O 7,10%; H2d 5,18%; H20− 0,99%; P2O5 0,01%; insgesamt 100,09%. Die differentiellen Wärme- und Infrarotabsorptionsanalysen waren ähnlich denen des Hydromuskowits. Das Röntgenbeugungsbild unterschied sich deutlich von jenen des IM und/oder 2M1 Polymorphs und war ähnlich dem des 2M2 Polymorphs, das bekanntlich in Lepidoliten vorkommt.
Абстракция
Гидромусковит в ассоциации с гипсом и ангидритом обнаружен в месторождении Шаканай, префектура Акита, Япония. Химический состав минерала: Si02-47, 14%; Ti02-0,34%; А12О3-37,09%; Ре2О3-0, 49%; МgО-0.83%; СаО-0, 57%; Na2O-0, 35%; K2O-7, 10%; Н2O+-5, 18%; Н2О—0, 99%; Р2О5-0,01%; сумма 100, 09%.
Данные дифференциально-термического анализа и инфракрасной спектроскопии сходны с результатами, полученными ранее для других гидромусковитов. Порошкограммы обнаруживают явное отличие от таковой полиморфных модификаций 1М и/или 2М1 и сходны с порошкограммами порошка полиморфной модификации 2М2, которая характерна для некоторых лепидолитов.
Similar content being viewed by others
References
Heinrich E. W., Levinson A. A., Levandowski D. W. and Hewitt C. H. (1953) Studies in the natural history of micas: University of Michigan Engineering Res. Inst. project M. 978.
Hendricks S. B. and Jefferson M. (1939) Polymorphism of the micas, with optical measurments: Am. Mineralogist 24, 729–771.
Levinson A. A. (1953) Studies in the mica group; Relationship between polymorphism and composition in the muscovite-lepidolite series: Am. Mineralogist 38, 88–107.
Oinuma K. and Hayashi H. (1968) Infrared spectra of clay minerals: J. Toyo Univ. General Education (Nat. Sci.) 9, 57–98.
Radoslovich E. W. (1960) Hydromuscovite with the 2M2 structure—A criticism: Am. Mineralogist 45, 894–898.
Shimoda S., Sudo T. and Oinuma K. (1969) Differential thermal analysis curves of mica clay minerals: Proc. Intern Clay Conf., Tokyo, Vol. 1, 197–206.
Smith J. V. and Yoder H. S. (1956) Experimental and theoretical studies of the mica polymorphs: Mineral. Mag. 31, 209–235.
Threadgold I. M. (1959) A hydromuscovite with the 2M2 structure, from Mount Lyell, Tasmania: Am. Mineralogist 44, 488–494.
Tornita K. and Sudo T. (1968) Interstratified structure formed from a pre-heated mica by acid treatments: Nature 217, 1043–1044.
Yoder H. S. and Eugster H. P. (1955) Synthetic and natural muscovite: Geochim. et Cosmochim. Acta 8, 225–280.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Shimoda, S. A Hydromuscovite from the Shakanai Mine, Akita Prefecture, Japan. Clays Clay Miner. 18, 269–274 (1970). https://doi.org/10.1346/CCMN.1970.0180505
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1970.0180505