Abstract
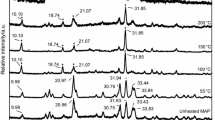
Changes in the infrared absorption spectrum of ammonium-saturated rectorite on heating suggest that the ammonium cations are hydrogen bonded to water molecules when the mineral is hydrated. Further spectral changes above 300°C indicate that lattice OH groups are perturbed by protons liberated from the decomposition of ammonium ions giving rise to an absorption doublet at 3500 and 3476 cm-1. The doublet attains maximal intensity when decomposition of ammonium cations and dehydroxylation of the mineral is complete at about 550°C.
The perturbation effect occurs only for swelling dioctahedral minerals which derive their layer charge from Al-for-Si substitution.
Similar content being viewed by others
References
Bokii, G. B., and Arkhipenko, D. K. (1962) Oxonium ions in vermiculite, Zh. Strukt. Khim. 3, 697–702.
Brindley, G. W. (1956) Allevardite, a swelling double-layer mica mineral, Amer. Min. 41, 91–103.
Brown, G., and Norrish, K. (1952) Hydrous micas, Mineral. Mag. 29, 929–32.
Brown, G., and Weir, A. H. (1963) The identity of rectorite and allevardite, Proc. Internat. Clay Conf., Stockholm, Pergamon Press, New York 1, 27–35.
Jorgensen, Per (1965) Infrared absorption of O—H bonds in some micas and other phyllosilicates, Clays and Clay Minerals, Proc. 13th Conf., Pergamon Press, New York (in press).
Korolev, Yu. M. (1960) The structure of allevardite, Kristallografiya 5, 891–5.
Ledoux, R. L., and White, J. L. (1964) Infrared study of selective deuteration of kaolinite and halloysite at room temperature, Science 145, 47–9.
Mortland, M. M., Fripiat, J. J., Chaussidon, J., and Uytterhoeven, J. B. (1963) Interaction between ammonia and the expanding lattices of montmorillonite and vermiculite, Jour. Phys. Chem. 67, 248–58.
Ockman, N. (1958) The infrared and raman spectra of ice, Advan. Phys. 7, 199–220.
Roy, D. M., and Roy, R. (1956) Hydrogen-deuterium exchange in clays and problems in the assignments of infrared frequencies in the hydroxyl region, Clays and Clay Minerals, Proc. 4th Conf., Natl. Acad. Sci.—Natl. Res. Council Pub. 456, pp. 82–4.
Russell, J. D., and Farmer, V. C. (1964) An infrared study of the dehydration of montmorillonite and saponite, Clay Min. Bull. 5, 443–64.
Uchiyama, N., and Onikura, Y. (1956) Double-layer mica minerals in paddy soil, Tôhoku Jour. Agr. Res. 7, 9–15.
Uytterhoeven, J. B., Christner, L. G., and Hall, W. K. (1965) Studies of the hydrogen held by solids. VIII. The decationated zeolites, Jour. Phys. Chem. 69, 2117–26.
White, J. L., and Burns, A. F. (1964) Infrared spectra of hydronium ion in micaceous minerals, Science 141, 800–1.
Author information
Authors and Affiliations
Additional information
This report is journal paper No. 2618 of the Purdue University Agricultural Experimental Station, Lafayette, Indiana.
On leave of absence from the Macaulay Institute for Soil Research, Aberdeen, Scotland.
Rights and permissions
About this article
Cite this article
Russell, J.D., White, J.L. Infrared Study of the Thermal Decomposition of Ammonium Rectorite. Clays Clay Miner. 14, 181–191 (1966). https://doi.org/10.1346/CCMN.1966.0140115
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1966.0140115