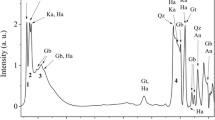
Abstract
The content of kaolinite plus halloysite is differentiated quantitatively from chlorite by differential dissolution, even in those samples wherein each mineral has 500°C heat instability of the 7 Å peak. This differentiation is possible because the structures of kaolinite and halloysite are destroyed on loss of the 7 Å peak, producing amorphous material which is rapidly soluble in 0.5 N NaOH, whereas the chlorite structure remains largely intact with only partial dehydration of the octahedral layer. The amorphous residue of kaolinite-halloysite is dissolved from the other mineral components of the heated sample with sodium hydroxide and the dissolved silica and alumina are allocated to kaolinite. The percentages of kaolinite thus obtained for Elliott, B3–C horizon, 2–0.2 μ fraction, are 3.8 and 3.7 based on silica and alumina, respectively. Similarly, the percentages of kaolinite obtained for Buchanan, B1 horizon, 2-0.2 μ fraction, are 14.9 and 13.8 based on silica and alumina, respectively. Collapse of the vermiculite basal spacing to 10 Å on K saturation and heating to 300°C, together with the clear differentiation of kaolinite by differential dissolution analysis (D.D.A.) above, removed the possibility of misinterpretation of the chlorite-like diffractometer tracing by kaolinite and vermiculite occurring together. Other clays, from Davidson and Susquehanna soils, had 44 and 42 to 43 percent kaolinite, based on silica and alumina, respectively. The vermiculite and montmoril-lonite contents of soil clay samples are quantitatively determined by specific surface. The mica percentages are based on the K2O after exclusion of potassium in feldspars. Water loss in the 400°C-to-ignition range gives an important quantitative basis for chlorite.
Similar content being viewed by others
References
Bradley, W. F. (1954) x-Ray diffraction criteria for the characterization of chloritic material in sediments: in Clays and Clay Minerals, Natl. Acad. Sci.–Natl. Res. Council pub, 327, pp. 324–334.
Bradley, W. F. (1958) Structural irregularities in hydrous magnesium silicates: in Clays and Clay Minerals, Natl. Acad. Sci.–Natl. Res. Council pub. 395, pp. 94–102.
Brindley, G. W. and Robinson, K. (1951) The chlorite minerals: in x-Ray Identification and Crystal Structure of Clay Minerals, Mineralogical Soc. London, pp. 173–198.
Brown, George (1953) The dioctahedral analogue of vermiculite: Clay Minerals Bull., v. 2, pp. 64–70.
Coleman, R. and Jackson, M. L. (1946) Mineral composition of the clay fraction of several coastal plain soils of southeastern United States: Soil Sci. Soc. Amer. Proc., v. 10, pp. 381–391.
Dixon, J. B. (1958) Mineralogical analyses of soil clays involving vermiculite-chlorite-kaolinite differentiation: PhD. thesis, University of Wisconsin, 76 pp.
Dixon, J. B. and Jackson, M. L. (1959) Dissolution of interlayers from intergradient soil clays after preheating at 400°C: Science, v. 129, pp. 1616–1617.
Dixon, J. B. and Jackson, M. L. (1960) Properties of intergradient chlorite-expansible layer silicates of soils: Soil Sci. Soc. Amer. Proc. (to be published).
Droste, J. B. (1956) Alteration of clay minerals by weathering in Wisconsin tills: Geol. Soc. Amer., Bull., v. 67, pp. 911–918.
Grim, R. E. (1953) Clay Mineralogy: McGraw-Hill Book Company, Inc., New York, 384 pp.
Hashimoto, I. and Jackson, M. L. (1960) Rapid dissolution of allophane and kaoluiite–halloysite after dehydration: in Clays and Clay Minerals, Pergamon Press, New York, pp. 102–113.
Jackson, M. L. (1956) Soil Chemical Analysis—Advanced Course: published by the author, Madison, Wisconsin, 991 pp.
Jackson, M. L. (1958) Soil Chemical Analysis: Prentice-Hall, Inc., Englewood Cliffs, N.J., 498 pp.
Jackson, M. L., Whittig, L. D., Vanden Heuvel, R. C., Kaufman, A. and Brown, B. E. (1954) Some analyses of soil montmorin, vermiculite, mica, chlorite, and mterstratified layer silicates: in Clays and Clay Minerals, Natl. Acad. Sci.–Natl. Res. Council pub. 327, pp. 218–240.
Jeffries, C. D. and Yearick, L. G. (1949) The essential mineral content of the principal soil series of Union County, Pennsylvania: Soil Sci. Soc. Amer. Proc, v. 13, pp. 146–152.
Kinter, E. B. and Diamond, S. (1958) Gravimetric determination of monolayer glycerol complexes of clay minerals: in Clays and Clay Minerals, Natl. Acad. Sci.–Natl. Res. Council pub. 566, pp. 318–333.
Martin, R. T. (1955) Reference chlorite characterization for chlorite identification in soil clays: in Clays and Clay Minerals, Natl. Acad. Sci.–Natl. Res. Council pub. 395, pp. 117–145.
Mehra, O. P. and Jackson, M. L. (1959) Constancy of the sum of mica unit cell potassium surface and interlayer sorption surface in vermiculite-illite clays; Soil Sci. Soc. Amer. Proc, v. 23, pp. 101–105.
Mehra, O. P. and Jackson, M. L. (1960) Iron oxide removal from soils and clays by a dithionite–citrate system buffered with sodium bicarbonate: in Clays and Clay Minerals, Pergamon Press, New York, pp. 317–327.
Pearson, R. W. and Ensminger, L. E. (1949) Types of clay minerals in Alabama soils: Soil Sci. Soc. Amer. Proc., v. 13, pp. 153–156.
Rich, C. I. and Obenshain, S. S. (1955) Chemical and clay mineral properties of a Red-Yellow Podzolic soil derived from muscovite schist: Soil Sci. Soc. Amer. Proc, v. 19, pp. 334–339.
Tamura, T. (1957) Identification of the 14Å clay mineral component: Amer. Min., v. 42, pp. 107–110.
Walker, G. F. (1957) On the differentiation of vermiculites and smectites in clays: Clay Minerals Bull., v. 3, pp. 154–163.
Whittig, L. D. and Jackson, M. L. (1955) Interstratified layer silicates in some soils in northern Wisconsin: in Clays and Clay Minerals, Natl. Acad. Sci.–Natl. Res. Council pub. 395, pp. 322–336.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dixon, J.B., Jackson, M.L. Mineralogical Analysis of Soil Clays Involving Vermiculite-Chlorite-Kaolinite Differentiation. Clays Clay Miner. 8, 274–286 (1959). https://doi.org/10.1346/CCMN.1959.0080124
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1959.0080124