Abstract
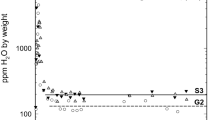
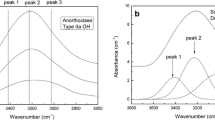
The problems of hydrous decomposition of alkali feldspars and the formation of the micaceous minerals from the decomposition products are discussed in terms of the surface chemistry and the Al-Si ordering of the feldspars, and a crystal growth model for the micas.
Similar content being viewed by others
References
Bailey, S. W. and Taylor, W. H. (1955) The structure of a triclinic potassium felspar: Acta Cryst., Camb., v. 8, pp. 621–632.
Bradley, W. F. (1940) The structural scheme of attapulgite: Amer. Min., v. 25, pp. 405–410.
Brauner, K. and Preisinger, A. (1956) Struktur und Entstehung des Sepioliths: Tschermaks mineral, petrog. Mitt., Bd. 6, pp. 120–140.
Brindley, G. W. and Radoslovich, W. W. (1956) x-ray studies of the alteration of soda feldspar: in Clays and Clay Minerals, Natl. Acad. Sci.-Natl. Res. Council, pub. 456, pp. 330–336.
DeVore, G. W. (1956a) Surface chemistry as a chemical control on mineral association: J. Geol., v. 64, pp. 31–55.
DeVore, G. W. (1956b) Al-Si positions in ordered plagioclase feldspar: Z. Kristallogr., v. 107, pp. 247–264.
DeVore, G. W. (1957) The association of strongly polarizing cations with weakly polarizing cations as a major influence on element distribution, mineral composition, and crystal growth: J. Geol., v. 65, pp. 178–195.
DeVore, G. W. (1958) A crystal growth model based on Al-Si ordering as an explanation for the polymorphism in the micas. (Z. Kristallogr., in press.)
Frederickson, A. F. and Cox, J. E. (1954a) The decomposition products of anorthite attacked by pure water at elevated temperatures and pressure, in Clays and, Clay Minerals, Natl. Acad. Sci.-Natl. Res. Council pub. 327, pp. 111–119.
Frederickson, A. F. and Cox, J. E. (1954b) “Solubility” of albite in hydrothermal solutions: Amer. Min., v. 39, pp. 738–749.
Frederiokson, A. F. and Cox, J. E. (1954c) Mechanism of “solution” of quartz in pure water at elevated temperatures and pressures: Amer. Min., v. 39, pp. 886–900.
Goldsmith, J. R. and Laves, Fritz (1954a) The microcline-sanidine stability relations: Geochim. Cosmochim. Acta., v. 5, pp. 1–19.
Goldsmith, J. R. and Laves, Fritz (1945b) Potassium feldspars structurally intermediate between mierocline and sanidine: Geochim. Cosmochim. Acta, v. 6, pp. 100–118.
Gruner, J. W. (1944) The hydrothermal alteration of feldspars in acid solutions between 300° and 400°C: Econ. Geol., v. 39, pp. 578–589.
Laves, Fritz (1951) Artificial preparation of microcline: J. Geol., v. 59, pp. 511–512.
Laves, Fritz (1952) Phase relations of the alkali feldspars: J. Geol., v. 60, pp. 549–574.
Laves, Fritz (1954) The coexistence of two plagioclases in the oligoclase compositional range: J. Geol., v. 62, pp. 409–411.
Morey, G. W. and Chen, W. T. (1955) The action of hot water on some feldspars: Amer. Min., v. 40, pp. 996–1000.
Mosebach, R. (1957) Thermodynamic behaviour of quartz and other forms of silica in pure water at elevated temperatures and pressures with conclusions on their mechanism of solution: J. Geol., v. 65, pp. 347–363.
Nagy, Bartholomew and Bradley, W. F. (1955) The structural scheme of sepiolite: Amer. Min., v. 40, pp. 885–892.
Steinfink, Hugo (1958a) The crystal structure of chlorite. I. A monoclinic polymorph: Acta Cryst., Camb., v. 11, pp. 191–195.
Steinfink, Hugo (1958b) The crystal structure of chlorite. II. A tricline polymorph: Acta Cryst., Camb., v. 11, pp. 195–198.
Taylor, W. H. (1933) The structure of sanidine and other feldspars: Z. Kristallogr., v. 85, pp. 435–442.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Devore, G.W. The Surface Chemistry of Feldspars as an Influence on Their Decomposition Products. Clays Clay Miner. 6, 26–41 (1957). https://doi.org/10.1346/CCMN.1957.0060104
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1957.0060104