Abstract
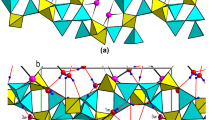
The crystal structure of cronstedtite-2H2 was refined in a hexagonal cell, space group P63, Z = 2, using two acicular crystals from Wheal Maudlin, Cornwall, England, and from Pribram, Czech Republic. The Wheal Maudlin sample has the chemical composition \(\left( {{\rm{Fe}}_{2.291}^{2 + }{\rm{Fe}}_{0.709}^{3 + }} \right)\left( {{\rm{S}}{{\rm{i}}_{1.298}}{\rm{Fe}}_{0.707}^{3 + }{\rm{A}}{{\rm{l}}_{0.004}}} \right){{\rm{O}}_5}{\left( {{\rm{OH}}} \right)_4}\) and the Příbram sample has the composition \(\left( {{\rm{Fe}}_{2.269}^{2 + }{\rm{Fe}}_{0.731}^{3 + }} \right)\left( {{\rm{S}}{{\rm{i}}_{1.271}}{\rm{Fe}}_{0.724}^{3 + }{\rm{A}}{{\rm{l}}_{0.005}}} \right){{\rm{O}}_5}{\left( {{\rm{OH}}} \right)_4}\). The results of refinements are as follows: a = 5.500(1), c = 14.163(2) Å, V = 371.08(8) Å3, R = 3.83%, from 381 independent reflections, and a = 5.4927(1), c = 14.1481(2) Å, V = 369.70(4) Å3, R = 4.77%, from 1088 independent reflections for the Wheal Maudlin and Příbram samples, respectively. The best Fovs. Fc agreement was achieved when the structure was interpreted as merohedral twin; several possible twinning laws are discussed. The cronstedtite layer consists of one tetrahedral sheet and one octahedral sheet. There is one octahedral (M1) position, occupied by Fe only, and two tetrahedral (T1, T2) positions in the structure. Refinement of occupancy of tetrahedral sites led to values Si:Fe = 0.45:0.55(1) (Wheal Maudlin) and 0.432:0.568(8) (Příbram) in T1, and Si: Fe = 0.99:0.01(1) (Wheal Maudlin) and 0.888:0.112(7) (Příbram) in 72. Whereas the size of T1 is reasonable (average dT1-O = 1.693 Å (Wheal Maudlin), 1.691 Å (Příbram)), T2 is unusually large: (dT2-O= 1.740 Å (Wheal Maudlin), 1.737 Å (Příbram)) with respect to the small or almost zero Fe content. As an explanation, an alternative structure model comprising a certain amount of vacancies in T2 is presented. The tetrahedral rotation angle α is highly positive (+12.1° and +12.5° for the Wheal Maudlin and Příbram samples, respectively), and the layer belongs to the Franzini type A. Distortion parameters of octahedra and tetrahedra are given for both samples. One hydrogen atom engaged in the hydrogen bond was located in the Wheal Maudlin sample.
Similar content being viewed by others
References
Allmann, R. and Donnay, G. (1973) The crystal structure of jugoldite. Mineralogical Magazine, 39, 271–281.
Anderson, C.S. and Bailey, S.W. (1981) A new cation ordering pattern in amesite-2H2American Mineralogist, 66, 185–195.
Angel, R.J. and Woodland, A.B. (1998) Crystal structure of spinelloid II in the system Fe3O4-Fe2SiO4. European Journal of Mineralogy, 10, 607–611.
Arakheeva, A.V. and Karpinskii, O.G. (1987) Crystal structure of the ternary hexagonal Ca ferrite Ca3.0Fe14.82O25. Kristallografiya, 32, 59–61 (in Russian).
Bailey, S.W. (1969) Polytypism of trioctahedral 1:1 layer silicates. Clays and Clay Minerals, 17, 355–371.
Bailey, S.W. (1988) Polytypism of 1:1 layer silicates. Pp. 9–27 in: Hydrous Phyllosilicates (Exclusive of Micas) (S.W. Bailey, editor). Reviews in Mineralogy, 19, Mineralogical society of America, Washington, D.C.
Bell, A.M.T. and Henderson, C.M.B. (1994) Rietveld refinement of the structures of dry-synthesized MFeIIISi2O6 leucites (M=K,Rb, Cs) by synchrotron X-ray powder diffraction. Acta Crystallographica, C50, 1531–1536.
Brigatti, M.F., Galli, E., Medici, L. and Poppi, L. (1997) Crystal structure of aluminian lizardite-2H2. American Mineralogist, 82, 931–935.
Brunton, G.D., Harris, L.A. and Kopp, O.C. (1972) Crystal structure of rubidium-iron feldspar. American Mineralogist, 57, 1720–1728.
Canillo, E., Mazzi, F., Fang, J.H., Robinson, P.D. and Ohya, Y. (1971) The crystal structure of aenigmatite. American Mineralogist, 56, 427–445.
Clegg, W. (1981) Faster data collection without loss of precision. An extension of the learnt profile method. Acta Crystallographica, A37, 22–28.
Collomb, A., Litsardakis, G., Samaras, D. and Pannetier, J. (1989) Neutron diffraction studies of the crystallographic and magnetic structures of SrZn2/3Mn4/3Fe16O27. Journal of Magnetism and Magnetic Materials, 78, 219–225.
de Boer, V., van Santen, J.H. and Verwey, E.J.W. (1950) The electrostatic contribution to the lattice energy of some ordered spinels. Journal of Chemical Physics, 18, 1032–1034.
Della Giusta, A., Princivalle, F. and Carbonin, S. (1987) Crystal structure and cation distribution in some natural magnetites. Mineralogy and Petrology, 37, 315–321.
Dollase, W.A. and Ross, C.R., II (1993) Crystal structure of the body centered tetragonal tectosilicates. American Mineralogist, 78, 627–632.
Donnay, G., Morimoto, N., Takeda, H. and Donnay, J.H.D. (1964) Trioctahedral one-layer micas. I. Crystal structure of a synthetic iron mica. Acta Crystallographica, 17, 1369–1373.
Dornberger-Schiff, K. and Ďurovič, S. (1975a) OD-interpretation of kaolinite-type structures — I: Symmetry of kaolinite packets and their stacking possibilities. Clays and Clay Minerals, 23, 219–229.
Dornberger-Schiff, K. and Ďurovič, S. (1975b) OD-interpretation of kaolinite-type structures — II: The regular polytypes (MDO-polytypes) and their derivation. Clays and Clay Minerals, 23, 231–246.
Dowty, E. (1991) ATOMS, a computer program for displaying structures. Shape Software, Kingsport, Tennessee.
Ďurovič, S. (1995) Troubles with cronstedtite-1M. Geologica Carpathica — Clays, 4, 88.
Ďurovič, S. (1997) Cronstedtite-1M and coexistence of 1M and 3T polytypes. Ceramics-Silikáty, 41, 98–104.
Franzini, M. (1969) The A and B mica layers and the crystal structure of sheet silicates. Contributions to Mineralogy and Petrology, 21, 203–224.
Geiger, C.A., Henry, D.L., Bailey, S.W. and Maj, J.J. (1983) Crystal structure of cronstedtite-2H2. Clays and Clay Minerals, 31, 97–108.
Giusepetti, G. and Tadini, C. (1972) The crystal structure of the 2O brittle mica anandite. Tschermaks Mineralogische und Petrographische Mitteilungen, 18, 169–184.
Grey, I.E., Hoskins, B.F. and Madsen, I.C. (1990) A structural study of the incorporation of silica into sodium ferrites, \({\rm{N}}{{\rm{a}}_{1 - x}}\left[ {{\rm{Fe}}_{1 - x}^{3 + }{\rm{S}}{{\rm{i}}_x}{{\rm{O}}_2}} \right]\), x = 0 to 0.20. Journal of Solid State Chemistry, 85, 202–219.
Guggenheim, S. and Eggleton, R.A. (1998) Modulated crystal structures of greenalite and caryopilite: A system with longrange, in-plane structural disorder in the tetrahedra sheet. The Canadian Mineralogist, 36, 163–179.
Guggenheim, S. and Zhan, W. (1998) Effect of temperature on the structures of lizardite-1T and lizardite-2H1. The Canadian Mineralogist, 36, 1587–1594.
Hall, S.H. and Bailey, S.W. (1979) Cation ordering pattern in amesite. Clays and Clay Minerals, 27, 241–247.
Hawthorne, F.C. (1978) The crystal chemistry of the amphiboles. VIII. The crystal structure and site chemistry of fluorriebeckite. The Canadian Mineralogist, 16, 187–194.
Hazen, R.M., Finger, L.W. and Velde, D. (1981) Crystal structure of a silica- and alkali-rich trioctahedral mica. American Mineralogist, 66, 586–591.
Hybler, J. (1997) Determination of crystal structures of minerals affected by twinning. PhD thesis, Charles University, Prague, Czech Republic, 138 pp. (in Czech with an English summary).
Hybler, J. (1998) Polytypism of cronstedtite from Chvaletice and Litošice. Ceramics-Silikáty, 42, 130–131.
Hybler, J., Petříček, V., Ďurovič, S. and Smrčok, L’. (2000) Refinement of the crystal structure of the cronstedtite-1T. Clays and Clay Minerals, 48, 331–338.
International Tables for Crystallography, Volume A (1983) D. Reidel Publishing Company, Dordrecht, The Netherlands.
International Tables for X-ray Crystallography, Volume IV (1974) The Kynoch Press, Birmingham, England.
Kato, T. (1986) The crystal structure of yeatmanite. Mineralogical Journal (Japan) 13, issue 2, 53–54.
Kogure, T., Hybler, J. and Ďurovič, S. (2001) A HRTEM study of cronstedtite: determination of polytypes and layer polarity in trioctahedral 1:1 phyllosilicates. Clays and Clay Minerals, 49, 310–317.
Konnert, J.A., Appleman, D.E., Clark, J.R., Finger, L.W., Kato, T. and Miura, Y. (1976) Crystal structure and cation distribution of hulsite, a tin-iron borate. American Mineralogist, 61, 116–122.
Ladd, M.F.C. and Palmer, R.A. (1977) Structure Determination by X-ray Crystallography. Plenum, New York, 393 pp.
Mellini, M. (1982) The crystal structure of lizardite-1T: hydrogen bonds and polytypism. American Mineralogist, 67, 587–598.
Mellini, M. and Viti, C. (1994) Crystal structure of lizardite-1T from Elba, Italy. American Mineralogist, 79, 1194–1198.
Mellini, M. and Zanazzi, P.F. (1987) Crystal structures of lizardite-1T and lizardite-2H1 from Coli, Italy. American Mineralogist, 72, 943–948.
Mellini, M., Weiss, Z., Rieder, M. and Drábek, M. (1996) Cs-ferrianite as a possible host of waste cesium. European Journal of Mineralogy, 8, 1265–1271.
Mereiter, K. (1978) Die Kristallstruktur des Voltaits, \({{\rm{K}}_2}{\rm{Fe}}_5^{2 + }{\rm{Fe}}_3^{3 + }{\rm{Al}}{\left( {{\rm{S}}{{\rm{O}}_{\rm{4}}}} \right)_{12}} \cdot 18\left( {{{\rm{H}}_{\rm{2}}}{\rm{O}}} \right)\)Tschermaks Mineralogische und Petrographische Mitteilungen, 18, 185–202.
Mikloš, D. (1975) Symmetry and polytypism of trioctahedral kaolin-type minerals. PhD thesis, Institute of Inorganic Chemistry, Slovak Academy of Sciences, Bratislava, Slovakia, 144 pp. (in Slovak).
Modaressi, A., Gerardin, R., Malaman, B. and Gleitzer, C. (1984) Structure et proprietes d’un germanate de fer de valence mixte Fe4Ge2O9. Etude succinte de GexFe(3−x)O4 (x < 0.5). Journal of Solid State Chemistry, 53, 22–24.
Palmer, D.C., Dove, M.T., Ibberson, R.M. and Powell, B.M. (1997) Structural behavior, crystal chemistry and phase transitions in substituted leucite: High resolution neutron powder diffraction studies. American Mineralogist, 82, 16–29.
Petříček, V. and Dušek, M. (2000) The crystallographic computing system JANA2000. Institute of Physics, Praha, Czech Republic.
Radoslovich, E.W. (1961) Surface symmetry and cell dimension of layer-lattice silicates. Nature, London, 191, 67–68.
Redhammer, G.J. (1998) Mössbauer spectroscopy and Rietveld refinement of synthetic ferri-Tschermak’s molecule CaFe3+(Fe3+Si)O6 substituted diopside. European Journal of Mineralogy, 10, 439–452.
Renner, B. and Lehmann, G. (1986) Correlation of angular and bond length distortions in TO4 units in crystals. Zeitschrift für Kristallographie, 175, 43–59.
Robinson, K., Gibbs, G.V. and Ribbe, P.H. (1971) Quadratic elongation: A quantitative measure of distortion in coordination polyhedra. Science, 172, 567–570.
Ross, C.R., Armbruster, T. and Canil, D. (1992) Crystal structure refinement of a spinelloid in the system Fe3O4-Fe2SiO4. American Mineralogist, 77, 507–511.
Semenova, T.F., Rozhdestvenskaya, I.V. and Frank-Kamenetskii, V.A. (1977) Refinement of the crystal structure of tetraferri phlogopite. Kristallografiya, 22, 1196–1201 (in Russian).
Smrčok, L’., Ďurovič, S., Petříček, V. and Weiss, Z. (1994) Refinement of the crystal structure of cronstedtite-3T. Clays and Clay Minerals, 42, 544–551.
Steadman, R. (1964) The structure of trioctahedral kaolin-type silicates. Acta Crystallographica, 17, 924–927.
Steadman, R. and Nuttall, P.M. (1963) Polymorphism in cronstedtite. Acta Crystallographica, 16, 1–8.
Steadman, R. and Nuttall, P.M. (1964) Further polymorphism in cronstedtite. Acta Crystallographica, 17, 404–406.
Steinfink, H. (1962) Crystal structure of a trioctahedral mica: Phlogopite. American Mineralogist, 47, 886–889.
Tagai, T. and Joswig, W. (1985) Untersuchungen der Kationverteilung im Staurolith durch Neutronenbeugung bei 100 K. Neues Jahbuch für Mineralogie Monatshefte, 97–107.
Taylor, H.F.W. (1992) Tobermorite, jennite and cement gel. Zeitschrift für Kristallographie, 202, 41–50.
Templeton, D.H. and Templeton, L.K. (1978) Program AGNOST C. University of California at Berkeley.
Toraya, H. (1981) Distortion of octahedra and octahedral sheets in 1M micas and the relation to their stability. Zeitschrift für Kristallographie, 157, 173–190.
Wechsler, B.A., Lindsley, D.H. and Prewitt, C.T. (1984) The crystal structure and cation distribution in titanomagnetites (Fe3−xTixO4). American Mineralogist, 69, 754–770.
Weiss, Z., Rieder, M., Chmielová, M. and Krajíček, J. (1985) Geometry of the octahedral coordination in micas. American Mineralogist, 70, 747–757.
Weiss, Z., Rieder, M. and Chmielová, M. (1992) Deformation of coordination polyhedra and their sheets in phyllosilicates. European Journal of Mineralogy, 4, 665–682.
Wiewióra, A., Rausell-Colom, J.A. and García-Gonzáles, T. (1991) The structure of amesite from Mount Sobotka: A nonstandard polytype. American Mineralogist, 76, 647–652.
Woodland, A.B. and Angel, R.J. (1998) Crystal structure of a new spinelloid with the wadsleyite structure in the system Fe2SiO4 − Fe3O4 and implications for the earth’s mantle. American Mineralogist, 83, 404–408.
Yakubovich, O.V., Simonov, M.A., Egorov-Tismenko, Y.K. and Belov, N.V. (1977) The crystal structure of a synthetic variety of alluadite. Doklady Akademii Nauk SSSR, 236, 1123–1126 (in Russian).
Zheng, H. and Bailey, S.W. (1997) Refinement of an amesite-2H1 polytype from Potmasburg, South Africa. Clays and Clay Minerals, 45, 301–310.
Zhukhlistov, A.P. and Zvyagin, B.B. (1998) Crystal structure of lizardite-1T from electron diffractometry data. Kristallographiya, 43, 1009–10014 (in Russian); also in: Crystallography Reports 43, 950–955.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hybler, J., Petříček, V., Fábry, J. et al. Refinement of the crystal structure of cronstedtite-2H2. Clays Clay Miner. 50, 601–613 (2002). https://doi.org/10.1346/000986002320679332
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1346/000986002320679332