Abstract
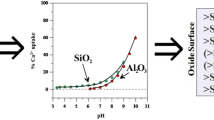
The cause of pH and ionic strength-dependent proton and hydroxyl adsorption onto kaolinite is specific binding at edge Al and Si sites, and it can be modeled as a function of temperature with a triple layer model (TLM) of the mineral-solution interface. Exchange of Al for protons and hydroxyls is observed at low pH, with a stoichiometry approaching 1:3 (Al:H+). Adsorption of organic acids from dilute solutions depends on: 1) solution pH; 2) the functionality of the acid; and, to a lesser extent, 3) temperature. Such adsorption may occur primarily at Al sites exposed on kaolinite edges, as indicated by sorption experiments on the constituent oxides, where negligible sorption was observed on SiO2 (quartz), but was significant on Al2O3 (corundum) surfaces. Under similar conditions, oxalate adsorbs more strongly than acetate or formate to aluminol sites.
Similar content being viewed by others
References
Balistrieri LS, Murray JW. 1981. The surface chemistry of goethite (alpha FeOOH) in major ion seawater Am J Sci 281:788–806.
Bethke CM. 1994. The geochemist’s workbench(™). A users guide to Rxn, Act2, Tact, React, and Gtplot. Champaign, IL: Univ of Illinois. 212 p.
Bolt G. 1957. Determination of the charge density of silica sols J Phys Chem 61:1166–1170.
Brady PV, Cygan RT, Nagy KL. 1996. Molecular controls on kaolinite surface charge. J Coll Interf Sci 183:183–364.
Brady PV, House WA. 1996. Surface-controlled dissolution and growth of minerals. In: Brady PV, editor. Physics and chemistry of mineral surfaces. Boca Raton, FL: CRC Pr., p 225–306.
Chan D, Perram JW, White LW. 1975. Regulation of surface potential at amphoteric surfaces during particle-particle interaction. J Chem Soc, Faraday Trans 71:1046–1057.
Davies CW. 1962. Ion association. Washington, DC: Butterworth. 180 p.
Davis JA, James RO, Leckie JO. 1978. Surface ionization and complexation at the oxide-water interface. 1. Computation of electrical double layer properties in simple electrolytes. J Colloid Interface Sci 63:63–499.
Davis JA, Kent DB. 1990. Surface complexation modeling in aqueous chemistry. In: Hochella MF, White AF, editors. Mineral-interface geochemistry. Washington, DC: Am Mineral Soc., p 177–260.
Dzombak DA, Morel FMM. 1990. Surface complexation modeling: Hydrous ferric oxide. New York: J. Wiley. 393 p.
Grandstaff DE. 1986. The dissolution rate of forsteritic olivine from Hawaiian beach sand. 3rd Int Water-Rock Interaction Symp. Geochem and Cosmochem Soc, Alberta Research Council, p 72–74.
Hayes KF, Redden GW, Leckie JO. 1990. Application of surface complexation models for radionuclide adsorption: Sensitivity analysis of model input parameters final report. Washington, DC: USNRC. 72 p.
Hayes KF, Redden GW, Leckie JO. 1991. Surface complexation models: An evaluation of model parameter estimation using FITEQL and oxide mineral titration data J Colloid Interface Sci 142:448–469.
Herbelin AL, Westall JC. 1994. FITEQL—A computer program for determination of chemical equilibrium constants from experimental data. Oregon State Univ Chem Dept.
Kettler RM, Palmer DA, Wesolowski DJ. 1991. Dissociation quotients of oxalic acid in aqueous sodium-chloride media to 175 degrees C. J Solution Chem 20:905–927.
Machesky ML. 1990. Influence of temperature on ion adsorption by hydrous metal oxides. In: Melchior DC, Bassett RL, editors. Chemical modeling in aqueous systems II. ACS Symp Series #416. Washington, DC: ACS. p 262–274.
Machesky ML, Jacobs PF. 1991. Titration calorimetry of aqueous alumina suspensions, Part II. Discussion of enthalpy changes with pH and ionic strength. Colloids and Surf 53:315–328.
Mast MA, Drever JI. 1987. The effect of oxalate on the dissolution rates of oligoclase and tremolite. Geochim Cosmochira Acta 51:51–2568.
Oelkers EH, Schott J, Devidal JL. 1994. The effect of aluminum, pH, and chemical affinity on the rates of aluminosilicate dissolution reactions. Geochim Cosmochim Acta 58:2011–2024.
Siffert B. 1962. Quelques reactions de la silice en solution: La formation des argiles. Mem Serv Carte Geol 21:21–86.
Small JS. 1992. Clay precipitation from oxalate-bearing solutions. In: Kharaka Y, Maest AS, editors. Water-rock interaction. Rotterdam: Balkema. p 345–348.
Sposito G. 1984. The surface chemistry of soils. New York: Oxford Univ Pr., 234 p.
Stumm W, Furrer G, Kunz B. 1983. The role of surface coordination in precipitation and dissolution of mineral phases. Croat Chim Acta 46:46–611.
Stumm W, Morgan JJ. 1981. Aquatic chemistry. New York: Wiley-Interscience. 780 p.
Tewari PH, McLean AW. 1972. Temperature dependence of point of zero charge of alumina and magnetite. J Colloid Interface Sci 40:40–272.
Van Den Vlekkert H, Bousse L, de Rooij N. 1988. The temperature dependence of the surface potential at the A1203/electrolyte interface. J Colloid Interface Sci 122:122–345.
Ward DB. 1995. Nickel adsorption on a natural sand and goethite, kaolinite, and quartz: Single- vs. multi-site models and the role of CO2. Albuquerque, NM: Univ of New Mexico. 304 p.
Weast RC. 1973. Handbook of chemistry and physics, 61st edition. Boca Raton, FL: CRC Pr., 2488 p.
Wesolowski DJ, Palmer DA. 1992. Aluminum speciation and equilibria in aqueous solution: V. Gibbsite solubility at 50°C and pH 3–9 in 0.1 molal NaCl solutions. Geochim Cosmochim Acta 58:58–2969.
Wieland E, Stumm W. 1992. Dissolution kinetics of kaolinite in acidic aqueous solutions at 25°C. Geochim Cosmochim Acta 56:56–3355.
Xie Z, Walther JV. 1992. Incongruent dissolution and surface area of kaolinite. Geochim Cosmochim Acta 56:56–3363.
Yates DE, Levine S, Healy TW. 1974. Site-binding model of the electrical double layer at the oxide/water interface. J Chem Soc London, Faraday Trans 70:1807–1818.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ward, D.B., Brady, P.V. Effect of Al and Organic Acids on the Surface Chemistry of Kaolinite. Clays Clay Miner. 46, 453–465 (1998). https://doi.org/10.1346/CCMN.1998.0460410
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1346/CCMN.1998.0460410