Summary
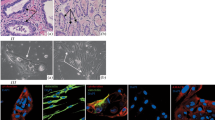
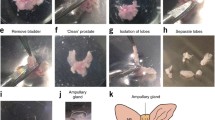
Three-dimensional epithelial culture models are widely used to emulate a more physiologically relevant microenvironment for the study of genes and signaling pathways. Prostate epithelial cells can grow into solid cell masses or acinus-like spheroids in Matrigel. To test if the ability to form acinus-like spheroids in Matrigel is dependent on how undifferentiated a cell is or whether it is tumor or nontumor, we established six novel epithelial cell lines. Primary prostate epithelial cells were immortalized using HPV16 E6 gene transduction and were named Shmac 2, 3, and 6 (nontumor); Shmac 4, Shmac 5, and P4E6 (tumor). All cell lines were phenotyped in monolayer culture, and their ability to form acinus-like spheroids in Matrigel investigated. The cell lines exhibited a wide range of population doubling times and all showed an intermediate phenotype in nonolayer culture (luminalCK+/basalCK+/CD44+/PSA+/AR−). Only Shmac 5 cells formed acinus-like spheroids when cultured in Matrigel. Co-culture of the spheroids with fibroblasts advanced differentiation by inducing androgen receptor expression and epithelial polarization. Our findings indicate that tumor cells can form acinus-like spheroids in Matrigel.
Similar content being viewed by others
References
Bae, V. L.; Jackson-Cook, C. K.; Maygarden, S. J.; Plymate, S. R.; Chen, J.; Ware, J. L. Metastatic sublines of an SV40 large T antigen immortalized human prostate epithelial cell line. Prostate 34:275–282; 1998.
Barcellos-Hoff, M. H.; Aggeler, J.; Ram, T. G.; Bissell, M. J. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development 105:223–235; 1989.
Bayne, C. W.; Donnelly, F.; Chapman, K.; Bollina, P.; Buck, C.; Habib, F. K. A novel coculture model for benign prostatic hyperplasia expressing both isoforms of 5a-reductase. J. Clin. Endocrinol. Metab. 83:206–213; 1998.
Bello-DeOcampo, D.; Kleinman, H. K.; Webber, M. M. The role of α6β1 integrin and EGF in normal and malignant acinar morphogenesis of human prostatic epithelial cells. Mutat. Res. 480–481:209–217; 2001.
Bemis, L. T.; Schedin, P. Reproductive state of rat mammary gland stroma modulates human breast cancer cell migration and invasion. Cancer Res. 60:3414–3418; 2000.
Collins, A. T.; Habib, F. K.; Maitland, N. J.; Neal, D. E. Identification and isolation of human prostate epithelial stem cells based on α2β1-integrin expression. J. Cell Sci. 114:3865–3872; 2001.
Darcy, K. M.; Zangani, D.; Shea-Eaton, W., et al. Mammary fibroblasts stimulate growth, alveolar morphogenesis, and functional differentiation of normal rat mammary epithelial cells. In Vitro Cell. Dev. Biol. 36A:578–592; 2000.
Debnath, J.; Muthuswamy, S. K.; Brugge, J. S. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30:256–268; 2003.
Hall, J. A.; Maitland, N. J.; Stower, M.; Lang, S. H. Primary prostate stromal cells modulate the morphology and migration of primary prostate epithelial cells in Type 1 collagen gels. Cancer Res. 62:58–62; 2002.
Jones, P. H.; Harper, S.; Watt, F. M. Stem cell patterning and fate in human epidermis. Cell 80:83–93; 1995.
Lang, S. H.; Hyde, C.; Reid, I. N.; Hitchcock, I. S.; Hart, C. A.; Bryden, A. A. G.; Villette, J.-M.; Stower, M. J.; Maitland, N. J. Enhanced expression of vimentin in motile prostate cell lines and in poorly differentiated and metastatic prostate cancer. Prostate 52:253–263; 2002.
Lang, S. H.; Sharrard, R. M.; Stark, M.; Villette, J. M.; Maitland, N. J. Prostate epithelial cell lines form spheroids with evidence of glandular differentiation in three-dimensional Matrigel cultures. Br. J. Cancer 85:590–599; 2001a.
Lang, S. H.; Stark, M.; Collins, A.; Paul, A. B.; Stower, M. J.; Maitland, N. J. Experimental prostate epithelial morphogenesis in response to stroma and three-dimensional matrigel culture. Cell Growth Differ. 12:631–640; 2001b.
Liu, A. Y.; True, L. D.; LaTray, L., et al. Cell-cell interaction in prostate gene regulation and cytodifferentiation. Proc. Natl. Acad. Sci. USA 94:10705–10710; 1997.
Maitland, N. J.; Macintosh, C. A.; Hall, J.; Sharrard, M.; Quinn, G.; Lang, S. In vitro models to study cellular differentiation and function in human prostate cancers. Radiat. Res. 155:133–142; 2001.
Maitland, N. J.; Macintosh, C. A.; Schmitz, C.; Lang, S. Immortalisation of human prostate cells with the human papillomavirus type 16 E6 gene. In: Langdon, S., ed. Cancer cell culture. Totowa, New Jersey: The Human Press; 2004:275–285.
Mitchell, S.; Abel, P.; Ware, M.; Stamp, G.; Lalani, E. N. Phenotypic and genotypic characterization of commonly used human prostatic cell lines. BJU Int. 85:932–944; 2000.
Muthuswamy, S. K.; Li, D.; Lelievre, S.; Bissell, M. J.; Brugge, J. S. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat. Cell Biol. 3:785–792; 2001.
Niemann, C.; Brinkmann, V.; Spitzer, E.; Hartmann, G.; Sachs, M.; Naundorf, H.; Birchmeier, W. Reconstitution of mammary gland development in vitro: requirement of c-met and c-erbB2 signaling for branching and alveolar morphogenesis. J. Cell Biol. 143:533–545; 1998.
O'Brien, L. C.; Zegers, M. M. P.; Mostov, K. E. Building epithelial architecture insights from three-dimensional culture models. Nat. Rev. Mol. Cell Biol. 3:531–537; 2002.
Park, C. C.; Zhang, H.; Pallavicini, M.; Gray, J. W.; Baehner, F.; Park, C. J.; Bissel, M. J. β1 integrin inhibitory antibody induces apoptosis of breast cancer cells, inhibits growth and distinguishes malignant from normal phenotype in three dimensional cultures and in vivo. Cancer Res. 66:1526–1535; 2006.
Richardson, G. D.; Robson, C. N.; Lang, S. H.; Neal, D. E.; Maitland, N. J.; Collins, A. T. CD133, a novel marker for human prostatic epithelial stem cells. J. Cell Sci. 117:3539–3545; 2004.
Robinson, E. J.; Neal, D. E.; Collins, A. T. Basal cells are progenitors of luminal cells in primary cultures of differentiating human prostatic epithelium. Prostate 37:149–160; 1998.
Schwab, T. S.; Stewart, T.; Lehr, J.; Pienta, K. J.; Rhim, J. S.; Macoska, J. A. Phenotypic characterization of immortalized normal and primary tumor-derived human prostate epithelial cell cultures. Prostate 44:164–171; 2000.
Sherwood, E. R.; Theyer G.; Steiner, G.; Berg, L. A.; Kozlowski, J. M.; Lee, C. Differential expression of specific cytokeratin polypeptides in the basal and luminal epithelia of the human prostate. Prostate 18:303–314; 1991.
Sommers, C. L.; Byers, S. W.; Thompson, E. W.; Torri, J. A.; Gelmann, E. P. Differentiation state and invasiveness of human breast cancer cell lines. Breast Cancer Res. Treatment 31:325–335; 1994.
Van Leenders, G.; Dijkman, H.; Hulsbergen-van de Kaa, C.; Ruiter, D.; Schalken, J. Demonstration of intermediate cells during human prostate epithelial differentiation in situ and in vitro using triple-staining confocal scanning microscopy. Lab. Invest. 80:1251–1258; 2000.
Varani, J.; Hattori, Y.; Dame, M. K.; Schmidt, T.; Murphy, H. S.; Johnson, K. J.; Wojno, K. J. Matrix metalloproteinases (MMPs) in fresh human prostate tumor tissue and organ cultured prostate tissue: levels of collagenolytic and gelatinolytic MMPs are low, variable and different in fresh tissue versus organ-cultured tissue. Br. J. Cancer 84:1076–1083; 2001.
Webber, M. M.; Bello, D.; Kleinman, H. K.; Hoffman, M. P. Acinar differentiation by non-malignant immortalized human prostatic epithelial cells and its loss by malignant cells. Carcinogenesis 18:1225–1231; 1997.
Webber, M. M.; Quader, S. T. A.; Kleinman, H. K.; Bello-Deocampo, D.; Storto, P. D.; Bice, G.; DeMendonca-Calaca, W.; Williams, D. E. Human cell lines as an invitro/in vivo model for prostate carcinogenesis and progression. Prostate 47:1–13; 2001.
Wernert, W.; Seitz, G. Immunohistochemical investigation of different cytokeratins and Vimentin in the prostate from the fetal period up to adulthood and in prostate carcinoma. Path. Res. Pract. 182:617–626; 1987.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lang, S.H., Smith, J., Hyde, C. et al. Differentiation of prostate epithelial cellcultures by materigel/stromal cell glandular reconstruction. In Vitro Cell.Dev.Biol.-Animal 42, 273–280 (2006). https://doi.org/10.1290/0511080.1
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1290/0511080.1