Abstract
Background
For cT2N0M0 esophageal adenocarcinomas, the effects of neoadjuvant chemoradiotherapy (NT) on surgical outcomes and the oncological benefits to the patients are debatable. In this study, we investigated the optimal management for cT2N0M0 adenocarcinoma (1) assessing the perioperative impact of NT on esophagectomy and (2) evaluating the oncologic effect of NT in a homogeneous group of patients with clinical stage IIA. We hypothesized that NT does not negatively affect perioperative outcomes and provides an oncologic benefit to selected patients with cT2N0M0 disease.
Methods
The National Cancer Database was queried (2010–2019) for patients with cT2N0M0 esophageal adenocarcinoma undergoing esophagectomy. After propensity-matching to adjust for differences in patient and tumor characteristics, we compared postoperative outcomes (logistic regression) and survival (Kaplan–Meier and Cox regression) among those who underwent NT vs upfront surgery (S).
Results
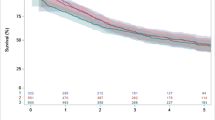
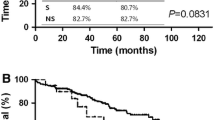
This study included 3413 patients, of whom 2359 (69%) received NT, and 1054 (31%) S. In contrast to those who underwent S, in the matched cohort, patients treated with NT had comparable conversion rates (8% vs11.1%, p = 0.06), length of stay (9 vs 10 days, p = 0.078), unplanned readmission (5.4% vs 8.8%, p = 0.109), and 30- (3.9% vs 3.7%, p = 0.90) and 90-day mortality (5.7% vs 4.7%, p = 0.599). In addition, NT associated with improved survival in patients with cT2N0M0 tumors > 5 cm (HR 0.30, 95% CI 0.17–0.36).
Conclusions
NT does not appear to increase technical complexity or to adversely affect postoperative outcomes after esophagectomy. Furthermore, minimally invasive esophagectomy is feasible following NT, with comparable conversion rates to those who had upfront surgery. Lastly, NT was selectively associated with improved survival in patients with cT2N0M0 esophageal cancer.





Similar content being viewed by others
References
van der Sluis PC, Schizas D, Liakakos T, van Hillegersberg R. Minimally invasive esophagectomy. Dig Surg. 2020;37(2):93–100. https://doi.org/10.1159/000497456.
Wei B, D’Amico TA. Thoracoscopic versus robotic approaches: advantages and disadvantages. Thoracic Surg Clin. 2014;24:177–88.
Zingg U, Smithers BM, Gotley DC, Smith G, Aly A, Clough A, et al. Factors associated with postoperative pulmonary morbidity after esophagectomy for cancer. Ann Surg Oncol. 2011;18:1460–8.
Shapiro J, Van Lanschot JJB, Hulshof MC, van Hagen P, van Berge Henegouwen MI, Wijnhoven BP, et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): long-term results of a randomised controlled trial. The Lancet Oncology. 2015;16:1090–8.
Tang H, Tan L, Shen Y, Wang H, Lin M, Feng M, et al. CMISG1701: a multicenter prospective randomized phase III clinical trial comparing neoadjuvant chemoradiotherapy to neoadjuvant chemotherapy followed by minimally invasive esophagectomy in patients with locally advanced resectable esophageal squamous cell carcinoma (cT3-4aN0-1M0)(NCT03001596). BMC Cancer. 2017;17:1–8.
Wang H, Tang H, Fang Y, Tan L, Yin J, Shen Y, et al. Morbidity and mortality of patients who underwent minimally invasive esophagectomy after neoadjuvant chemoradiotherapy vs neoadjuvant chemotherapy for locally advanced esophageal squamous cell carcinoma: a randomized clinical trial. JAMA Surgery. 2021;156:444–51.
Kim JY, Hofstetter WL. Esophagectomy after chemoradiation: who and when to operate. Semin Thoracic Cardiovasc Surg. 2012;24:288–93. https://doi.org/10.1053/j.semtcvs.2012.10.005.
Franko J, Voynov G, Goldman CD. Esophagectomy timing after neoadjuvant therapy for distal esophageal adenocarcinoma. Ann Thoracic Surg. 2016;101:1123–30.
Berlth F, Hadzijusufovic E, Mann C, Fetzner UK, Grimminger P. Minimal invasive Ösophagektomie für maligne Tumore der Speiseröhre. Therapeutische Umschau. 2022;79:181–7.
Mitzman B, Lutfi W, Wang C-H, Krantz S, Howington JA, Kim K-W. Minimally Invasive Esophagectomy Provides Equivalent Survival to Open Esophagectomy: An Analysis of the National Cancer Database. Sem Thoracic Cardiovasc Surg. 2017;29:244–53. https://doi.org/10.1053/j.semtcvs.2017.03.007.
Tapias LF, Mathisen DJ, Wright CD, Wain JC, Gaissert HA, Muniappan A, et al. Outcomes with open and minimally invasive Ivor Lewis esophagectomy after neoadjuvant therapy. Ann Thoracic Surg. 2016;101:1097–103.
Bograd AJ, Molena D. Minimally invasive esophagectomy. Curr Probl Surg. 2021;58:100984–100984.
Mariette C, Markar SR, Dabakuyo-Yonli TS, Meunier B, Pezet D, Collet D, et al. Hybrid minimally invasive esophagectomy for esophageal cancer. N Engl J Med. 2019;380:152–62.
Kamel MK, Sholi AN, Rahouma M, Harrison SW, Lee B, Stiles BM, et al. National trends and perioperative outcomes of robotic oesophagectomy following induction chemoradiation therapy: a National Cancer Database propensity-matched analysis. Eur J Cardio-Thoracic Surg. 2021;59:403–8.
Halpern AL, Friedman C, Torphy RJ, Al-Musawi MH, Mitchell JD, Scott CD, et al. Conversion to open surgery during minimally invasive esophagectomy portends worse short-term outcomes: an analysis of the National Cancer Database. Surg Endosc. 2020;34:3470–8. https://doi.org/10.1007/s00464-019-07124-y.
Ben-David K, Rossidis G, Zlotecki RA, Grobmyer SR, Cendan JC, Sarosi GA, et al. Minimally invasive esophagectomy is safe and effective following neoadjuvant chemoradiation therapy. Ann Surg Oncol. 2011;18:3324–9.
Sihag S, Kosinski AS, Gaissert HA, Wright CD, Schipper PH. Minimally invasive versus open esophagectomy for esophageal cancer: a comparison of early surgical outcomes from the society of thoracic surgeons national database. Ann Thorac Surg. 2016;101:1281–9. https://doi.org/10.1016/j.athoracsur.2015.09.095.
Zhang JQ, Hooker CM, Brock MV, Shin J, Lee S, How R, et al. Neoadjuvant chemoradiation therapy is beneficial for clinical stage T2 N0 esophageal cancer patients due to inaccurate preoperative staging. Ann Thorac Surg. 2012;93:429–37.
Stiles BM, Mirza F, Coppolino A, Port JL, Lee PC, Paul S, et al. Clinical T2–T3N0M0 esophageal cancer: the risk of node positive disease. Ann Thorac Surg. 2011;92:491–8. https://doi.org/10.1016/j.athoracsur.2011.04.004.
Deng W, Yang J, Ni W, Li C, Chang X, Han W, et al. Postoperative radiotherapy in pathological T2–3N0M0 thoracic esophageal squamous cell carcinoma: interim report of a prospective, phase III, randomized controlled study. The Oncologist. 2020;25:e701–8.
Denlinger CS, Matkowskyj KA, Mulcahy MF. Gastric and esophageal cancers: guidelines updates. JNCCN J Natl Compr Cancer Netw. 2021;19:639–43.
Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15:683–90.
Huang Y, Chang J, Guo X, Zhang C, Ji W, Zhou S, et al. Induction chemotherapy increases efficacy and survival rate of patients with locally advanced esophageal squamous cell carcinoma. Front Oncol. 2022;12:1067838. https://doi.org/10.3389/fonc.2022.1067838.
Vadhwana B, Zosimas D, Lykoudis P, Phen H, Martinou M, Khoo D. Tumour length as an independent prognostic factor in resectable oesophageal carcinoma. Annals. 2020;102:185–90. https://doi.org/10.1308/rcsann.2019.0147.
Song Z, Wang J, Lin B, Zhang Y. Analysis of the tumor length and other prognosis factors in pT1-2 node-negative esophageal squamous cell carcinoma in a Chinese population. World J Surg Oncol. 2012;10:273. https://doi.org/10.1186/1477-7819-10-273.
Griffiths EA, Brummell Z, Gorthi G, Pritchard SA, Welch IM. Tumor length as a prognostic factor in esophageal malignancy: Univariate and multivariate survival analyses. J Surg Oncol. 2006;93:258–67. https://doi.org/10.1002/jso.20449.
Yendamuri S, Swisher SG, Correa AM, Hofstetter W, Ajani JA, Francis A, et al. Esophageal tumor length is independently associated with long-term survival. Cancer. 2009;115:508–16. https://doi.org/10.1002/cncr.24062.
Mariette C, Dahan L, Mornex F, Maillard E, Thomas P-A, Meunier B, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. JCO. 2014;32:2416–22. https://doi.org/10.1200/JCO.2013.53.6532.
Mu Y, Wang H, He T, Xu L. The impact of radiation dose on preoperative neoadjuvant chemoradiotherapy effects for patients with locally advanced squamous cell esophageal carcinoma: a propensity score-matched retrospective study. J Immunol Res. 2022;2022:7581799. https://doi.org/10.1155/2022/7581799.
Liu X-H, Hu Y, Li K-K, Wang Y-J, Jiang Y-G, Guo W. Intraoperative conversion does not affect the oncological outcomes of minimally invasive esophagectomy for treatment of esophageal cancer. Surg Endosc. 2018;32:4517–26.
Sancheti M, Fernandez F. Management of T2 esophageal cancer. Surg Clin. 2012;92:1169–78.
Speicher PJ, Ganapathi AM, Englum BR, Hartwig MG, Onaitis MW, D’Amico TA, et al. Induction therapy does not improve survival for clinical stage T2N0 esophageal cancer. J Thorac Oncol. 2014;9:1195–201. https://doi.org/10.1097/JTO.0000000000000228.
Perez Holguin RA, Olecki EJ, Stahl KA, Wong WG, Vining CC, Dixon MEB, et al. Management of clinical T2N0 esophageal and gastroesophageal junction adenocarcinoma: What is the optimal treatment? J Gastrointest Surg. 2022;26:2050–60. https://doi.org/10.1007/s11605-022-05441-7.
Funding
This research did not receive financial support from any source.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Stiles: Medtronic, AstraZeneca, Genentech, Pfizer, Arcus Biosciences, Bristol Myers Squib, BMS Foundation, Gala Therapeutics, Merck, and the Lung Cancer Research Foundation. The rest of the authors have no conflicts of interest to disclose.
Ethical Approval
This research was performed following the 1964 Helsinki Declaration and its later amendments and was approved by an institutional review board. This work has not been published elsewhere.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10434_2023_14441_MOESM2_ESM.jpg
Supplementary Fig. 1. Sensitivity analysis to determine the tumor size cutoff for subgroups in the survival analysis (JPG 28 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rodriguez-Quintero, J.H., Kamel, M.K., Jindani, R. et al. The Effect of Neoadjuvant Therapy on Esophagectomy for cT2N0M0 Esophageal Adenocarcinoma. Ann Surg Oncol 31, 228–238 (2024). https://doi.org/10.1245/s10434-023-14441-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14441-z