Abstract
Background
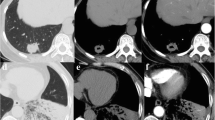
Primary lung mucinous adenocarcinomas (LMAs) could be subclassified as the pure-solid, part-solid, and pneumonic types according to the findings of high-resolution computed tomography. This study aimed to expound on the clinicopathologic, radiologic, and prognostic characteristics of LMAs based on radiologic classification within a large set of patients.
Methods
From November 2009 to December 2016, this study enrolled 294 resected LMAs, which were divided into the pure-solid (n = 169), part-solid (n = 87), and pneumonic (n = 38) types. The clinicopathologic and radiologic characteristics of the tumors were evaluated, and patient prognosis was determined through follow-up evaluation. Survival outcomes were calculated by Kaplan-Meier curves and compared using log-rank tests. The prognostic impact of clinicopathologic variables, including radiologic presentations, were evaluated by establishing a Cox proportional hazards model.
Results
The LMAs were infrequently associated with lymph node metastasis (5.4 %), lymphatic/vascular invasion (4.4 %), or visceral pleural invasion (5.1 %). During the median 71-month follow-up period, recurrence was observed in 62 patients and death in 44 patients. The patients with pneumonic-type LMAs had a poorer prognosis (5-year recurrence-free survival [RFS], 23.7 %; 5-year overall survival [OS], 44.7 %) than those with the pure-solid type (RFS, 83.2 %; OS, 100 %) or part-solid type (RFS, 93.7 %; OS, 100 %). Besides, lymph node metastasis, emphysema, and clinical T stage were independent predictors of RFS and OS.
Conclusion
Solitary-type LMA patients had excellent prognoses, whereas the survival outcomes for pneumonic-type LMA patients were dismal. Furthermore, pneumonic-type LMA patients were prone to intrapulmonary metastasis by means of aerogenous dissemination rather than distant metastasis.




Similar content being viewed by others
References
Ichinokawa H, Ishii G, Nagai K, et al. Clinicopathological characteristics of primary lung adenocarcinoma predominantly composed of goblet cells in surgically resected cases. Pathol Int. 2011;61:423–9.
Travis WD, Brambilla E, Noguchi M, et al. International association for the study of lung cancer/American Thoracic Society/European Respiratory Society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol. 2011;6:244–85.
Lee HY, Lee KS, Han J, et al. Mucinous versus nonmucinous solitary pulmonary nodular bronchioloalveolar carcinoma: CT and FDG PET findings and pathologic comparisons. Lung Cancer. 2009;65:170–5.
Lee HY, Cha MJ, Lee KS, et al. Prognosis in resected invasive mucinous adenocarcinomas of the lung: related factors and comparison with resected nonmucinous adenocarcinomas. J Thorac Oncol. 2016;11:1064–73.
Boland JM, Maleszewski JJ, Wampfler JA, et al. Pulmonary invasive mucinous adenocarcinoma and mixed invasive mucinous/nonmucinous adenocarcinoma: a clinicopathological and molecular genetic study with survival analysis. Hum Pathol. 2018;71:8–19.
Shim HS, Kenudson M, Zheng Z, et al. Unique genetic and survival characteristics of invasive mucinous adenocarcinoma of the lung. J Thorac Oncol. 2015;10:1156–62.
Geles A, Gruber-Moesenbacher U, Quehenberger F, et al. Pulmonary mucinous adenocarcinomas: architectural patterns in correlation with genetic changes, prognosis, and survival. Virchows Arch. 2015;467:675–86.
Watanabe H, Saito H, Yokose T, et al. Relation between thin-section computed tomography and clinical findings of mucinous adenocarcinoma. Ann Thorac Surg. 2015;99:975–81.
Miyata N, Endo M, Nakajima T, et al. High-resolution computed tomography findings of early mucinous adenocarcinomas and their pathologic characteristics in 22 surgically resected cases. Eur J Radiol. 2015;84:993–7.
Shimizu K, Okita R, Saisho S, Maeda A, et al. Clinicopathological and immunohistochemical features of lung invasive mucinous adenocarcinoma based on computed tomography findings. Onco Targets Ther. 2017;10:153–63.
Wang T, Yang Y, Liu X, et al. Primary invasive mucinous adenocarcinoma of the lung: prognostic value of CT imaging features combined with clinical factors. Korean J Radiol. 2021;22:652–62.
Aquino SL, Chiles C, Halford P, et al. Distinction of consolidative bronchioloalveolar carcinoma from pneumonia: Do CT criteria work? AJR Am J Roentgenol. 1998;171:359–63.
Jung JI, Kim H, Park SH, et al. CT differentiation of pneumonic-type bronchioloalveolar cell carcinoma and infectious pneumonia. Br J Radiol. 2001;74:490–4.
Gaikwad A, Souza CA, Inacio JR, et al. Aerogenous metastases: a potential game changer in the diagnosis and management of primary lung adenocarcinoma. AJR Am J Roentgenol. 2014;203:W570–82.
Cha YJ, Kim HR, Lee HJ, Cho BC, et al. Clinical course of stage IV invasive mucinous adenocarcinoma of the lung. Lung Cancer. 2016;102:82–8.
Gaeta M. Patterns of recurrence of bronchioloalveolar cell carcinoma after surgical resection: a radiological, histological, and immunohistochemical study. Lung Cancer. 2003;42:319–26.
Oka S, Hanagiri T, Uramoto H, et al. Surgical resection for patients with mucinous bronchioloalveolar carcinoma. Asian J Surg. 2010;33:89–93.
Moon SW, Choi SY, Moon MH, et al. Effect of invasive mucinous adenocarcinoma on lung cancer-specific survival after surgical resection: a population-based study. J Thorac Dis. 2018;10:3595–608.
Cha YJ, Shim HS. Biology of invasive mucinous adenocarcinoma of the lung. Transl Lung Cancer Res. 2017;6:508–12.
Zwirewich CV, Vedal S, Miller RR, Müller NL. Solitary pulmonary nodule: high-resolution CT and radiologic-pathologic correlation. Radiology. 1991;179:469–76.
Lee KH, Goo JM, Park SJ, et al. Correlation between the size of the solid component on thin-section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground-glass nodules. J Thorac Oncol. 2014;9:74–82.
Yoshizawa A, Motoi N, Riely GJ, et al. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol. 2011;24:653–64.
Warth A, Muley T, Meister M, et al. The novel histologic international association for the study of lung cancer/American thoracic society/European respiratory society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J Clin Oncol. 2012;30:1438–46.
Matsui T, Sakakura N, Koyama S, et al. Comparison of surgical outcomes between invasive mucinous and non-mucinous lung adenocarcinoma. Ann Thorac Surg. 2021;112:1118–26.
Ichinokawa H, Ishii G, Nagai K, et al. Distinct clinicopathologic characteristics of lung mucinous adenocarcinoma with KRAS mutation. Hum Pathol. 2013;44:2636–42.
Sakuma Y, Matsukuma S, Yoshihara M, et al. Distinctive evaluation of nonmucinous and mucinous subtypes of bronchioloalveolar carcinomas in EGFR and K-ras gene-mutation analyses for Japanese lung adenocarcinomas: confirmation of the correlations with histologic subtypes and gene mutations. Am J Clin Pathol. 2007;128:100–8.
Kadota K, Yeh YC, D’Angelo SP, et al. Associations between mutations and histologic patterns of mucin in lung adenocarcinoma: invasive mucinous pattern and extracellular mucin are associated with KRAS mutation. Am J Surg Pathol. 2014;38:1118–27.
Finberg KE, Sequist LV, Joshi VA, et al. Mucinous differentiation correlates with absence of EGFR mutation and presence of KRAS mutation in lung adenocarcinomas with bronchioloalveolar features. J Mol Diagn. 2007;9:320–6.
Miller VA, Hirsch FR, Johnson DH, et al. Systemic therapy of advanced bronchioloalveolar cell carcinoma: challenges and opportunities. J Clin Oncol. 2005;23:3288–93.
De Perrot M, Chernenko S, Waddell TK, et al. Role of lung transplantation in the treatment of bronchogenic carcinomas for patients with end-stage pulmonary disease. J Clin Oncol. 2004;22:4351–6.
Rami-Porta R, Bolejack V, Crowley J, et al. The IASLC lung cancer staging project: proposals for the revisions of the T descriptors in the forthcoming eighth edition of the TNM classification for lung cancer. J Thorac Oncol. 2015;10:990–1003.
Zhang J, Gold KA, Lin HY, et al. Relationship between tumor size and survival in non-small cell lung cancer (NSCLC): an analysis of the surveillance, epidemiology, and end results (SEER) registry. J Thorac Oncol. 2015;10:682–90.
Acknowledgment
The authors gratefully acknowledge the help of Mr. Huikang Xie (Department of Pathology, Shanghai Pulmonary Hospital, Tongji University School of Medicine), who kindly evaluated the pathologic retrospective data.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, W., Yang, Y., Yang, M. et al. Clinicopathologic Features and Survival Outcomes of Primary Lung Mucinous Adenocarcinoma Based on Different Radiologic Subtypes. Ann Surg Oncol 31, 167–177 (2024). https://doi.org/10.1245/s10434-023-14193-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-14193-w