Abstract
Background
Intrahepatic cholangiocarcinoma (iCCA) has a high recurrence rate and poor prognosis, and chemotherapy options are limited. The prevalence of cancer-associated fibroblasts (CAFs) in iCCA has recently emerged as a prognostic marker and therapeutic target. A method to quantify the expression of CAFs is needed; however, a simple and reliable quantification method has not yet been established.
Objective
The aim of this study was to establish a simple and reliable method of quantifying CAFs.
Methods
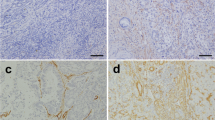
A total of 71 patients with iCCA who underwent curative resection from November 2006 to October 2020 in our hospital were investigated. Immunohistochemistry for alpha-smooth muscle actin (α-SMA) was performed and α-SMA-positive cells were quantified by an automated analysis system (new method) and visually counted (conventional method). The times required for measurement and the prognosis were compared.
Results
The results of the quantification of CAFs by the new method were significantly correlated with the results by the conventional method, and the time required for measurement was significantly shorter with the new method. Patients with high-intensity CAFs showed a significantly poorer prognosis in terms of overall survival (OS) and the cumulative hepatic recurrence rate. In addition, high α-SMA levels were a significant risk factor for OS in multivariate analysis.
Conclusions
This new method may contribute to the management of patients with iCCA, not only for the prediction of prognosis of patients with iCCA, but also for the indication of targeted therapy against CAFs.



Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, Shogo Kobayashi, upon reasonable request.
References
Buettner S, van Vugt JL, Groot Koerkamp B. Intrahepatic cholangiocarcinoma: current perspectives. Onco Targets Ther. 2017;10:1131–42.
Sha M, Jeong S, Qiu BJ, et al. Isolation of cancer-associated fibroblasts and its promotion to the progression of intrahepatic cholangiocarcinoma. Cancer Med. 2018;7(9):4665–77.
Konstantinidis IT, Groot Koerkamp B, Do RK, et al. Unresectable intrahepatic cholangiocarcinoma: systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer. 2016;122(5):758–65.
Gao Q, Zhao YJ, Wang XY, et al. Activating mutations in PTPN3 promote cholangiocarcinoma cell proliferation and migration and are associated with tumor recurrence in patients. Gastroenterology. 2014;146(5):1397–407.
Xie P, Guo L, Zhang B, et al. Case report: immunotherapy successfully treated brain metastasis in intrahepatic cholangiocarcinoma and literature review. Front Oncol. 2022;12:911202.
Van Cutsem E, Tempero MA, Sigal D, et al. Randomized phase III trial of pegvorhyaluronidase alfa with nab-paclitaxel plus gemcitabine for patients with hyaluronan-high metastatic pancreatic adenocarcinoma. J Clin Oncol. 2020;38(27):3185–94.
Kocher HM, Basu B, Froeling FEM, et al. Phase I clinical trial repurposing all-trans retinoic acid as a stromal targeting agent for pancreatic cancer. Nat Commun. 2020;11(1):4841.
Sirica AE. The role of cancer-associated myofibroblasts in intrahepatic cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 2011;9(1):44–54.
Yamanaka T, Harimoto N, Yokobori T, et al. Nintedanib inhibits intrahepatic cholangiocarcinoma aggressiveness via suppression of cytokines extracted from activated cancer-associated fibroblasts. Br J Cancer. 2020;122(7):986–94.
Joshi RS, Kanugula SS, Sudhir S, Pereira MP, Jain S, Aghi MK. The role of cancer-associated fibroblasts in tumor progression. Cancers (Basel). 2021;13(6):1399.
Kobayashi H, Enomoto A, Woods SL, Burt AD, Takahashi M, Worthley DL. Cancer-associated fibroblasts in gastrointestinal cancer. Nat Rev Gastroenterol Hepatol. 2019;16(5):282–95.
Okabe H, Beppu T, Hayashi H, et al. Hepatic stellate cells may relate to progression of intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2009;16(9):2555–2264.
Chuaysri C, Thuwajit P, Paupairoj A, Chau-In S, Suthiphongchai T, Thuwajit C. Alpha-smooth muscle actin-positive fibroblasts promote biliary cell proliferation and correlate with poor survival in cholangiocarcinoma. Oncol Rep. 2009;21(4):957–69.
Gabasa M, Ikemori R, Hilberg F, Reguart N, Alcaraz J. Nintedanib selectively inhibits the activation and tumour-promoting effects of fibroblasts from lung adenocarcinoma patients. Br J Cancer. 2017;117(8):1128–38.
Thongchot S, Vidoni C, Ferraresi A, et al. Cancer-associated fibroblast-derived IL-6 determines unfavorable prognosis in cholangiocarcinoma by affecting autophagy-associated chemoresponse. Cancers (Basel). 2021;13(9):2134.
Mertens JC, Fingas CD, Christensen JD, et al. Therapeutic effects of deleting cancer-associated fibroblasts in cholangiocarcinoma. Cancer Res. 2013;73(2):897–907.
Mukai Y, Yamada D, Eguchi H, et al. Vitamin D supplementation is a promising therapy for pancreatic ductal adenocarcinoma in conjunction with current chemoradiation therapy. Ann Surg Oncol. 2018;25(7):1868–79.
Ramos-Vega V, Venegas Rojas B, Donoso Torres W. Immunohistochemical analysis of cancer-associated fibroblasts and podoplanin in head and neck cancer. Med Oral Patol Oral Cir Bucal. 2020;25(2):e268–76.
Zou R, Jiang Q, Jin T, Chen M, Yao L, Ding H. Pan-cancer analyses and molecular subtypes based on the cancer-associated fibroblast landscape and tumor microenvironment infiltration characterization reveal clinical outcome and immunotherapy response in epithelial ovarian cancer. Front Immunol. 2022;13:956224.
Iseki Y, Shibutani M, Maeda K, et al. A new method for evaluating tumor-infiltrating lymphocytes (TILs) in colorectal cancer using hematoxylin and eosin (H-E)-stained tumor sections. PLoS One. 2018;13(4):e0192744.
Teng F, Mu D, Meng X, et al. Tumor infiltrating lymphocytes (TILs) before and after neoadjuvant chemotherapy and its clinical utility in rectal cancer. Am J Cancer Res. 2015;5:2064–74.
Hu B, Wu C, Mao H, et al. Subpopulations of cancer-associated fibroblasts link the prognosis and metabolic features of pancreatic ductal adenocarcinoma. Ann Transl Med. 2022;10(5):262.
Author information
Authors and Affiliations
Contributions
SE, DY, SK, HT, TN, TA, MT, YD made substantial contributions to the conception and design, acquisition of data, analysis and interpretation of data, drafted the manuscript, and gave final approval of the version to be published. YI made substantial contributions to the conception and design, acquisition of data, revised the manuscript critically for important intellectual content, and gave final approval of the version to be published. YT made substantial contributions to the conception and design, acquisition of data, revised the manuscript critically for important intellectual content, and gave final approval of the version to be published. HE made substantial contributions to the conception and design, analysis and interpretation of data, revised the manuscript critically for important intellectual content, and gave final approval of the version to be published.
Corresponding author
Ethics declarations
Disclosures
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Eguchi, S., Yamada, D., Kobayashi, S. et al. Automated Analysis for the Prevalence of Cancer-Associated Fibroblasts in Resected Specimens of Intrahepatic Cholangiocarcinoma is a Simple and Reliable Evaluation System. Ann Surg Oncol 30, 5420–5428 (2023). https://doi.org/10.1245/s10434-023-13633-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13633-x