Abstract
Background
Three-course neoadjuvant chemotherapy (NAC) followed by surgery has become a standard of care for locally advanced esophageal cancer (EC). However, some patients occasionally experience a poor tumor response to the third course and have a poor clinical outcome.
Methods
An exploratory analysis of data from the authors’ recent multicenter randomized phase 2 trial compared patients with locally advanced EC who received two courses (n = 78) and those who received three courses (n = 68) of NAC. The association between tumor response and clinico-pathologic factors, including survival, was evaluated to identify risk factors in the three-course group.
Results
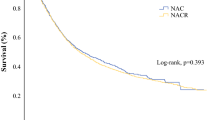
Of 68 patients who received three courses of NAC, 28 (41.2%) had a tumor reduction rate lower than 10% during the third course. This rate was associated with unfavorable overall survival (OS) and progression-free survival (PFS) compared with a tumor reduction rate of 10% or higher (2-year OS rate: 63.5% vs. 89.3%, P = 0.007; 2-year PFS rate: 52.6% vs. 79.7%, P = 0.020). The independent prognostic factors for OS were tumor reduction rate lower than 10% during the third course (hazard ratio [HR], 2.735; 95% confidence interval [CI] 1.041–7.188; P = 0.041) and age of 65 years or older (HR, 9.557, 95% CI 1.240–73.63; P = 0.030). Receiver operating characteristic curve and multivariable logistic regression analyses identified a tumor reduction rate lower than 50% after the first two courses as an independent predictor of a tumor reduction rate lower than 10% during the third course of NAC (HR, 4.315; 95% CI 1.329–14.02; P = 0.015).
Conclusion
Continuing NAC through a third course may worsen survival for patients who do not experience a response to the first two courses in locally advanced EC.




Similar content being viewed by others
References
Watanabe M, Tachimori Y, Oyama T, et al. Comprehensive registry of esophageal cancer in Japan, 2013. Esophagus. 2021;18:1–24.
Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019;16:1–24.
Ajani JA, D’Amico TA, Bentrem DJ, et al. Esophageal and esophagogastric junction cancers, version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2019;17:855–83.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68–74.
Yamasaki M, Yasuda T, Yano M, et al. Multicenter randomized phase II study of cisplatin and fluorouracil plus docetaxel (DCF) compared with cisplatin and fluorouracil plus adriamycin (ACF) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (OGSG1003). Ann Oncol. 2017;28:116–20.
Shiraishi O, Yamasaki M, Makino T, et al. Feasibility of preoperative chemotherapy with docetaxel, cisplatin, and 5-fluorouracil versus adriamycin, cisplatin, and 5-fluorouracil for resectable advanced esophageal cancer. Oncology. 2017;92:101–8.
Makino T, Yamasaki M, Miyazaki Y, et al. Utility of initial induction chemotherapy with 5-fluorouracil, cisplatin, and docetaxel (DCF) for T4 esophageal cancer: a propensity score-matched analysis. Dis Esophagus. 2018;31:dox130.
Urakawa S, Makino T, Yamasaki M, et al. Lymph node response to neoadjuvant chemotherapy as an independent prognostic factor in metastatic esophageal cancer. Ann Surg. 2021;273:1141–9.
Hagi T, Makino T, Yamasaki M, et al. Pathological regression of lymph nodes better predicts long-term survival in esophageal cancer patients undergoing neoadjuvant chemotherapy followed by surgery. Ann Surg. 2022;275:1121–9.
Kato K, Ito Y, Daiko H, et al. A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. In: 2022 ASCO Gastrointestinal Cancers Symposium; 2022. p. 238.
Hara J, Miyata H, Yamasaki M, et al. Mesenchymal phenotype after chemotherapy is associated with chemoresistance and poor clinical outcome in esophageal cancer. Oncol Rep. 2014;31:589–96.
Kubo Y, Tanaka K, Masuike Y, et al. Low mitochondrial DNA copy number induces chemotherapy resistance via epithelial-mesenchymal transition by DNA methylation in esophageal squamous cancer cells. J Transl Med. 2022;20:383.
Makino T, Yamasaki M, Tanaka K, et al. Multicenter randomised trial of two versus three courses of preoperative cisplatin and fluorouracil plus docetaxel for locally advanced oesophageal squamous cell carcinoma. Br J Cancer. 2022;126:1555–62.
Shiraishi O, Makino T, Yamasaki M, et al. Two versus three courses of preoperative cisplatin and fluorouracil plus docetaxel for treating locally advanced esophageal cancer: short-term outcomes of a multicenter randomized phase II trial. Esophagus. 2021;18:825–34.
Sobin LH, Wittekind C. TNM classification of malignant tumors. 7th edn. Oxford: Wiley-Blackwell; 2010.
Makino T, Yamasaki M, Miyata H, et al. Solitary lymph node recurrence of esophageal squamous cell carcinoma: surgical failure or systemic disease? Ann Surg Oncol. 2016;23:2087–93.
Kubo Y, Miyata H, Sugimura K, et al. Prognostic implication of postoperative weight loss after esophagectomy for esophageal squamous cell cancer. Ann Surg Oncol. 2021;28:184–93.
Kubo Y, Tanaka K, Yamasaki M, et al. Influences of the Charlson Comorbidity Index and nutrition status on prognosis after esophageal cancer surgery. Ann Surg Oncol. 2021;28:7173–82.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Motoori M, Yano M, Yasuda T, et al. Early response to neoadjuvant chemotherapy in advanced esophageal cancer evaluated by computed tomography predicts the utility of a second cycle of chemotherapy. Mol Clin Oncol. 2013;1:521–6.
Matsumoto S, Wakatsuki K, Nakade H, et al. Impact of CT-assessed changes in tumor size after neoadjuvant chemotherapy on pathological response and survival of patients with esophageal squamous cell carcinoma. Langenbecks Arch Surg. 2022;407:965–74.
Makino T, Yamasaki M, Miyata H, et al. p53 Mutation status predicts pathological response to chemoradiotherapy in locally advanced esophageal cancer. Ann Surg Oncol. 2010;17:804–11.
Makino T, Yamasaki M, Tanaka K, et al. Importance of positron emission tomography for assessing the response of primary and metastatic lesions to induction treatments in T4 esophageal cancer. Surgery. 2017;162:836–45.
Makino T, Yamasaki M, Tanaka K, et al. Metabolic tumor volume change predicts long-term survival and histological response to preoperative chemotherapy in locally advanced esophageal cancer. Ann Surg. 2019;270:1090–5.
Hashimoto T, Makino T, Yamasaki M, et al. The pattern of residual tumor after neoadjuvant chemotherapy for locally advanced esophageal cancer and its clinical significance. Ann Surg. 2020;271:875–84.
Makino T, Yamasaki M, Takeno A, et al. Cytokeratins 18 and 8 are poor prognostic markers in patients with squamous cell carcinoma of the oesophagus. Br J Cancer. 2009;101:1298–306.
Makino T, Yamasaki M, Takemasa I, et al. Dickkopf-1 expression as a marker for predicting clinical outcome in esophageal squamous cell carcinoma. Ann Surg Oncol. 2009;16:2058–64.
Makino T, Doki Y, Miyata H, et al. Use of (18)F-fluorodeoxyglucose-positron emission tomography to evaluate responses to neo-adjuvant chemotherapy for primary tumor and lymph node metastasis in esophageal squamous cell carcinoma. Surgery. 2008;144:793–802.
Noma T, Makino T, Ohshima K, et al. Immunoscore signatures in surgical specimens and tumor-infiltrating lymphocytes in pretreatment biopsy predict treatment efficacy and survival in esophageal cancer. Ann Surg. 2023;277:e528–37. https://doi.org/10.1097/SLA.0000000000005104.
Cunningham D, Allum WH, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20.
Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–57.
Sugimura K, Miyata H, Tanaka K, et al. Multicenter Randomized Phase 2 Trial Comparing chemoradiotherapy and docetaxel plus 5-fluorouracil and cisplatin chemotherapy as initial induction therapy for subsequent conversion surgery in patients with clinical T4b esophageal cancer: short-term results. Ann Surg. 2021;274:e465–72.
Akita H, Doki Y, Miyata H, et al. Clinical significance of the second-cycle response to cisplatin-based chemotherapy as preoperative treatment for esophageal squamous cell carcinoma. J Surg Oncol. 2006;93:401–9.
Kamarajah SK, Griffiths EA. Postoperative and pathological outcomes of CROSS and FLOT as neoadjuvant therapy for esophageal and junctional adenocarcinoma: an international cohort study from the Oesophagogastric Anastomosis Audit (OGAA). Ann Surg. 2022. https://doi.org/10.1097/SLA.0000000000005394.
Donlon NE, Moran B, Kamilli A, et al. CROSS versus FLOT regimens in esophageal and esophagogastric junction adenocarcinoma: a propensity-matched comparison. Ann Surg. 2022;276:792–8.
Mathieu A, Remmelink M, D’Haene N, et al. Development of a chemoresistant orthotopic human nonsmall cell lung carcinoma model in nude mice: analyses of tumor heterogenity in relation to the immunohistochemical levels of expression of cyclooxygenase-2, ornithine decarboxylase, lung-related resistance protein, prostaglandin E synthetase, and glutathione-S-transferase-alpha (GST)-alpha, GST-mu, and GST-pi. Cancer. 2004;101:1908–18.
Masuike Y, Tanaka K, Makino T, et al. Esophageal squamous cell carcinoma with low mitochondrial copy number has mesenchymal and stem-like characteristics, and contributes to poor prognosis. PLoS ONE. 2018;13:e0193159.
Smith BN, Bhowmick NA. Role of EMT in metastasis and therapy resistance. J Clin Med. 2016;5:17.
Mak MP, Tong P, Diao L, et al. A patient-derived, pan-cancer EMT signature identifies global molecular alterations and immune target enrichment following epithelial-to-mesenchymal transition. Clin Cancer Res. 2016;22:609–20.
Shibue T, Weinberg RA. EMT, CSCs, and drug resistance: the mechanistic link and clinical implications. Nat Rev Clin Oncol. 2017;14:611–29.
Momose K, Yamasaki M, Tanaka K, et al. MLH1 expression predicts the response to preoperative therapy and is associated with PD-L1 expression in esophageal cancer. Oncol Lett. 2017;14:958–64.
Hashimoto T, Kurokawa Y, Takahashi T, et al. Predictive value of MLH1 and PD-L1 expression for prognosis and response to preoperative chemotherapy in gastric cancer. Gastric Cancer. 2019;22:785–92.
Sargent DJ, Marsoni S, Monges G, et al. Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer. J Clin Oncol. 2010;28:3219–26.
Vilar E, Gruber SB. Microsatellite instability in colorectal cancer-the stable evidence. Nat Rev Clin Oncol. 2010;7:153–62.
Doki Y, Ajani JA, Kato K, et al. Nivolumab combination therapy in advanced esophageal squamous cell carcinoma. N Engl J Med. 2022;386:449–62.
Acknowledgement
The authors sincerely appreciate all participating investigators for their valuable assistance with data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10434_2023_13548_MOESM2_ESM.pdf
Fig. S2. Survival with tumor reduction (cutoff, 10%) during the third course of neoadjuvant chemotherapy by age (cutoff, 65 years). (PDF 32 kb)
10434_2023_13548_MOESM3_ESM.pdf
Fig. S3. Receiver operating characteristic curve analysis of the reduction rate after the first two courses of neoadjuvant chemotherapy that predicted a tumor reduction rate lower than 10% during the third course of neoadjuvant chemotherapy. (PDF 19 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kubo, Y., Makino, T., Yamasaki, M. et al. Three-Course Neoadjuvant Chemotherapy Associated with Unfavorable Survival of Non-responders to the First Two Courses for Locally Advanced Esophageal Cancer. Ann Surg Oncol 30, 5899–5907 (2023). https://doi.org/10.1245/s10434-023-13548-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-023-13548-7