Abstract
Background
Little is known about the survival impacts of pretreatment cancerous stenosis on patients with esophageal carcinoma (EC).
Methods
The clinicopathologic characteristics of patients who underwent surgery for EC between January 2010 and December 2018 were retrospectively reviewed. Esophageal stenosis was defined as present when a thin endoscope could not be passed through the tumor site. The impacts of stenosis on overall survival (OS) and cancer-specific survival (CSS) were evaluated using Cox hazards analysis.
Results
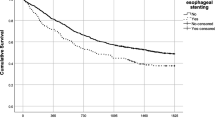
Of the 496 EC patients in this study, 51 (10.3 %) had pretreatment esophageal stenosis. Stenosis was associated with lower body mass index (P < 0.001) and higher pStage (P < 0.001). The 3-year OS rate for the patients with stenosis was significantly poorer than for the patients without stenosis (40.2 % vs 69.6 %; hazard ratio [HR], 2.19; P < 0.001). The survival outcomes, especially CSS, for the patients with stenosis were significantly poorer than for the patients without stenosis for both pStage II-III (P = 0.009) and pStage IV (P = 0.006) disease. The OS and CSS curves were well stratified by the presence of stenosis even in early-stage (pStage II) patients (P = 0.04 and P < 0.01, respectively). Multivariable analysis showed esophageal stenosis, pStage III-IV disease, and non-curative resection to be independently associated with poor OS (HR, 1.61; P = 0.02) and poor CSS (HR,1.67; P = 0.02). Higher pStage was an independent predictor of poor CSS for patients without stenosis, but not for those with stenosis.
Conclusions
Esophageal carcinoma patients with pretreatment stenosis had significantly poorer survival outcomes, especially poorer CSS, than those without stenosis in both early- and advanced-stage diseases.


Similar content being viewed by others
References
Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology, and risk factors. Clin J Gastroenterol. 2020;13:1010–21.
Smyth EC, Lagergren J, Fitzgerald RC, et al. Oesophageal cancer. Nat Rev Dis Primers. 2017;3:17048.
Hagi T, Makino T, Yamasaki M, et al. Dysphagia score as a predictor of adverse events due to triplet chemotherapy and oncological outcomes in 434 consecutive patients with esophageal cancer. Ann Surg Oncol. 2019;26:4754–64.
Deng HY, Alai G, Luo J, Li G, Zhuo ZG, Lin YD. Cancerous esophageal stenosis before treatment was significantly correlated to poor prognosis of patients with esophageal cancer: a meta-analysis. J Thorac Dis. 2018;10:4212–9.
Steenhagen E. Preoperative nutritional optimization of esophageal cancer patients. J Thorac Dis. 2019;11(Suppl 5):S645–53.
Ahmed O, Lee JH, Thompson CC, Faulx A. AGA clinical practice update on the optimal management of the malignant alimentary tract obstruction: expert review. Clin Gastroenterol Hepatol. 2021;19:1780–8.
Clavier JB, Antoni D, Atlani D, et al. Baseline nutritional status is prognostic factor after definitive radiochemotherapy for esophageal cancer. Dis Esophagus. 2014;27:560–7.
Okuno T, Wakabayashi M, Kato K, et al. Esophageal stenosis and the Glasgow Prognostic Score as independent factors of poor prognosis for patients with locally advanced unresectable esophageal cancer treated with chemoradiotherapy (exploratory analysis of JCOG0303). Int J Clin Oncol. 2017;22:1042–9.
Yang YS, Hu WP, Ni PZ, Wang WP, Yuan Y, Chen LQ. Esophageal luminal stenosis is an independent prognostic factor in esophageal squamous cell carcinoma. Oncotarget. 2017;8:43397–405.
du Rieu MC, Filleron T, Beluchon B, et al. Recurrence risk after Ivor Lewis oesophagectomy for cancer. J Cardiothorac Surg. 2013;8:215.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83.
Brierley J, Gospodarowicz M, Wittekind C. The TNM Classification of Malignant tumours. 8th ed. John Wiley & Sons, 2017.
Sugawara K, Aikou S, Yajima S, et al. Pre-and post-operative low prognostic nutritional index influences survival in older patients with gastric carcinoma. J Geriat Oncol. 2020;11(6):989–96.
Sugawara K, Mori K, Yagi K, Aikou S, Uemura Y, Yamashita H, Seto Y. Association of preoperative inflammation-based prognostic score with survival in patients undergoing salvage esophagectomy. Dis Esophagus. 2019;32(4):doy066.
Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017 edited by the Japan Esophageal Society: part 1. Esophagus. 2019;16:1–24.
Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68–74.
Hara H, Tahara M, Daiko H, et al. Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1455–60.
Mariette C, Gronnier C, Duhamel A, et al. Self-expanding covered metallic stent as a bridge to surgery in esophageal cancer: impact on oncologic outcomes. J Am Coll Surg. 2015;220:287–96.
Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48.
Kita R, Miyata H, Sugimura K, et al. Clinical effect of enteral nutrition support during neoadjuvant chemotherapy on the preservation of skeletal muscle mass in patients with esophageal cancer. Clin Nutr. 2021;40:4380–5.
Rodrigues-Pinto E, Ferreira-Silva J, Sousa-Pinto B, et al. Self-expandable metal stents in esophageal cancer before preoperative neoadjuvant therapy: efficacy, safety, and long-term outcomes. Surg Endosc. 2021;35:5130–9.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Shinoda M, Ando N, Kato K, et al. Randomized study of low-dose versus standard-dose chemoradiotherapy for unresectable esophageal squamous cell carcinoma (JCOG0303). Cancer Sci. 2015;106:407–12.
Sugawara K, Yamashita H, Okumura Y, Yagi K, Yoshimura S, Kawasaki K, Seto Y. Relationships among body composition, muscle strength, and sarcopenia in esophageal squamous cell carcinoma patients. Support Care Cancer. 2020;28(6):2797–803.
Sugawara K, Yamashita H, Okumura Y, Yagi K, Aikou S, Seto Y. Age-dependent survival impact of body mass index in patients with oesophageal squamous cell carcinoma. Eur J Surg Oncol. 2020;46(10 Pt A):1948–55.
Imamura H, Nishikawa K, Kishi K, et al. Effects of an oral elemental nutritional supplement on post-gastrectomy body weight loss in gastric cancer patients: a randomized controlled clinical trial. Ann Surg Oncol. 2016;23:2928–35.
Movahed S, Varshoee Tabrizi F, Pahlavani N, et al. Comprehensive assessment of nutritional status and nutritional-related complications in newly diagnosed esophageal cancer patients: a cross-sectional study. Clin Nutr. 2021;40:4449–55.
Previtali P, Fiore M, Colombo J, et al. Malnutrition and perioperative nutritional support in retroperitoneal sarcoma patients: results from a prospective study. Ann Surg Oncol. 2020;27:2025–32.
Mariette C, Balon JM, Maunoury V, Taillier G, Van Seuningen I, Triboulet JP. Value of endoscopic ultrasonography as a predictor of long-term survival in oesophageal carcinoma. Br J Surg. 2003;90:1367–72.
Morgan MA, Twine CP, Lewis WG, et al. Prognostic significance of failure to cross esophageal tumors by endoluminal ultrasound. Dis Esophagus. 2008;21:508–13.
Okadome K, Baba Y, Yagi T, et al. Prognostic nutritional index, tumor-infiltrating lymphocytes, and prognosis in patients with esophageal cancer. Ann Surg. 2020;271:693–700.
Xie SH, Santoni G, Mälberg K, Lagergren P, Lagergren J. Prediction model of long-term survival after esophageal cancer surgery. Ann Surg. 2021;273:933–9.
Sugawara K, Yagi K, Uemura Y, et al. Associations of systemic inflammation and sarcopenia with survival of esophageal carcinoma patients. Ann Thorac Surg. 2020;110:374–82.
Sugawara K, Yagi K, Okumura Y, Aikou S, Yamashita H, Seto Y. Survival prediction capabilities of preoperative inflammatory and nutritional status in esophageal squamous cell carcinoma patients. World J Surg. 2022;46:639–47.
Sugawara K, Yagi K, Okumura Y, et al. Long-term outcomes of multimodal therapy combining definitive chemoradiotherapy and salvage surgery for T4 esophageal squamous cell carcinoma. Int J Clin Oncol. 2020;25:552–60.
Yokota T, Kato K, Hamamoto Y, et al. Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer. 2016;115:1328–34.
Terada M, Hara H, Daiko H, et al. Phase III study of tri-modality combination therapy with induction docetaxel plus cisplatin and 5-fluorouracil versus definitive chemoradiotherapy for locally advanced unresectable squamous-cell carcinoma of the thoracic esophagus (JCOG1510: TRIANgLE). Jpn J Clin Oncol. 2019;49:1055–60.
Kato K, Ito Y, Daiko H, et al. A randomized controlled phase III trial comparing two chemotherapy regimen and chemoradiotherapy regimen as neoadjuvant treatment for locally advanced esophageal cancer, JCOG1109 NExT study. J Clin Oncol. 2022;40(4 Suppl):238–238.
Al-Batran SE, Homann N, Pauligk C, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019;393:1948–57.
Kato K, Cho BC, Takahashi M, et al. Nivolumab versus chemotherapy in patients with advanced oesophageal squamous cell carcinoma refractory or intolerant to previous chemotherapy (ATTRACTION-3): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20:1506–17.
Elliott JA, Doyle SL, Murphy CF, et al. Sarcopenia: prevalence, and impact on operative and oncologic outcomes in the multimodal management of locally advanced esophageal cancer. Ann Surg. 2017;266:822–30.
Deftereos I, Kiss N, Isenring E, Carter VM, Yeung JM. A systematic review of the effect of preoperative nutrition support on nutritional status and treatment outcomes in upper gastrointestinal cancer resection. Eur J Surg Oncol. 2020;46:1423–34.
Tanaka Y, Takeuchi H, Nakashima Y, et al. Effects of an elemental diet to reduce adverse events in patients with esophageal cancer receiving docetaxel/cisplatin/5-fluorouracil: a phase III randomized controlled trial-EPOC 2 (JFMC49-1601-C5). ESMO Open. 2021;6:100277.
Cepeda MS, Boston R, Farrar JT, Strom BL. Comparison of logistic regression versus propensity score when the number of events is low and there are multiple confounders. Am J Epidemiol. 2003;158:280–7.
Hwang ES, Wang X. Value of propensity score-matching to study surgical outcomes. Ann Surg. 2017;265:457–8.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
10434_2022_12945_MOESM3_ESM.tiff
Fig. S1 Survival outcomes according to the presence of stenosis and Glasgow prognostic score (GPS). (a) Overall survival (OS) and (b) cancer-specific survival (CSS) curves according to the presence of stenosis and GPS. Patients with both pretreatment stenosis and high GPS had much poorer survival outcomes (median survival time (MST), 5.9 months) than those who had only one or neither of these factors (P < 0.01).
Supplementary file3 (TIFF 8660 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sugawara, K., Fukuda, T., Kishimoto, Y. et al. The Impact of Pretreatment Esophageal Stenosis on Survival of Esophageal Cancer Patients. Ann Surg Oncol 30, 2703–2712 (2023). https://doi.org/10.1245/s10434-022-12945-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12945-8