Abstract
Background
High-volume centers (HVC), academic centers (AC), and longer travel distances (TD) have been associated with improved outcomes for patients undergoing surgery for pancreatic adenocarcinoma (PAC). Effects of mediating variables on these associations remain undefined. The purpose of this study is to examine the direct effects of hospital volume, facility type, and travel distance on overall survival (OS) in patients undergoing surgery for PAC and characterize the indirect effects of patient-, disease-, and treatment-related mediating variables.
Patients and Methods
Using the National Cancer Database, patients with non-metastatic PAC who underwent resection were stratified by annual hospital volume (< 11, 11–19, and ≥ 20 cases/year), facility type (AC versus non-AC), and TD (≥ 40 versus < 40 miles). Associations with survival were evaluated using multiple regression models. Effects of mediating variables were assessed using mediation analysis.
Results
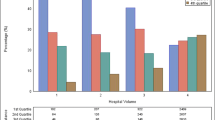
In total, 19,636 patients were included. Treatment at HVC or AC was associated with lower risk of death [hazard ratio (HR) 0.90, confidence interval (CI) 0.88–0.92; HR 0.89, CI 0.86–0.91, respectively]. TD did not impact OS. Patient-, disease-, and treatment-related variables explained 25.5% and 41.8% of the survival benefit attained from treatment at HVC and AC, reducing the survival benefit directly attributable to each variable to 4.9% and 6.4%, respectively.
Conclusions
Treatment of PAC at HVC and AC was associated with improved OS, but the magnitude of this benefit was less when mediating variables were considered. From a healthcare utilization and cost–resource perspective, further research is needed to identify patients who would benefit most from selective referral to HVC or AC.



Similar content being viewed by others
References
Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280(20):1747–51.
Birkmeyer JD, Siewers AE, Finlayson EV, et al. Hospital volume and surgical mortality in the United States. N Engl J Med. 2002;346(15):1128–37.
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33.
Liu Q, Zhao Z, Zhang X, et al. Perioperative and oncological outcomes of robotic versus open pancreaticoduodenectomy in low-risk surgical candidates: a multicenter propensity score-matched study. Ann Surg. 2021.
Zureikat AH, Beane JD, Zenati MS, et al. 500 minimally invasive robotic pancreatoduodenectomies: one decade of optimizing performance. Ann Surg. 2021;273(5):966–72.
Hue JJ, Sugumar K, Markt SC, et al. Facility volume–survival relationship in patients with early-stage pancreatic adenocarcinoma treated with neoadjuvant chemotherapy followed by pancreatoduodenectomy. Surgery. 2021;170(1):207–14.
Merath K, Mehta R, Tsilimigras DI, et al. In-hospital mortality following pancreatoduodenectomy: a comprehensive analysis. J Gastrointest Surg. 2020;24(5):1119–26.
Kagedan DJ, Goyert N, Li Q, et al. The impact of increasing hospital volume on 90-day postoperative outcomes following pancreaticoduodenectomy. J Gastrointest Surg. 2017;21(3):506–15.
O’Mahoney PRA, Yeo HL, Sedrakyan A, et al. Centralization of pancreatoduodenectomy a decade later: impact of the volume–outcome relationship. Surgery. 2016;159(6):1528–38.
Krautz C, Nimptsch U, Weber GF, Mansky T, Grützmann R. Effect of hospital volume on in-hospital morbidity and mortality following pancreatic surgery in Germany. Ann Surg. 2018;267(3):411–7.
Ryan CE, Wood TW, Ross SB, Smart AE, Sukharamwala PB, Rosemurgy AS. Pancreaticoduodenectomy in Florida: do 20-year trends document the salutary benefits of centralization of care? HPB (Oxford). 2015;17(9):832–8.
Maegawa FB, Ashouri Y, Bartz-Kurycki M, et al. Impact of facility type on survival after pancreatoduodenectomy for small pancreatic adenocarcinoma (≤ 2 cm). Am J Surg. 2021;222(1):145–52.
Watson MD, Miller-Ocuin JL, Driedger MR, et al. Factors associated with treatment and survival of early stage pancreatic cancer in the era of modern chemotherapy: an analysis of the National Cancer Database. J Pancreat Cancer. 2020;6(1):85–95.
Lidsky ME, Sun Z, Nussbaum DP, Adam MA, Speicher PJ, Blazer DG 3rd. Going the extra mile: improved survival for pancreatic cancer patients traveling to high-volume centers. Ann Surg. 2017;266(2):333–8.
Wasif N, Chang YH, Pockaj BA, Gray RJ, Mathur A, Etzioni D. Association of distance traveled for surgery with short- and long-term cancer outcomes. Ann Surg Oncol. 2016;23(11):3444–52.
Hunger R, Mantke R. Outcome quality beyond the mean - an analysis of 43,231 pancreatic surgical procedures related to hospital volume. Ann Surg. 2022;276:159–66.
Fry BT, Smith ME, Thumma JR, Ghaferi AA, Dimick JB. Ten-year trends in surgical mortality, complications, and failure to rescue in medicare beneficiaries. Ann Surg. 2020;271(5):855–61.
Healy MA, Krell RW, Abdelsattar ZM, et al. Pancreatic resection results in a statewide surgical collaborative. Ann Surg Oncol. 2015;22(8):2468–74.
Binkley CE, Kemp DS. Ethical centralization of high-risk surgery requires racial and economic justice. Ann Surg. 2020;272(6):917–8.
Blanco BA, Kothari AN, Blackwell RH, et al. “Take the Volume Pledge” may result in disparity in access to care. Surgery. 2017;161(3):837–45.
Bliss LA, Yang CJ, Chau Z, et al. Patient selection and the volume effect in pancreatic surgery: unequal benefits? HPB (Oxford). 2014;16(10):899–906.
Zafar SN, Shah AA, Channa H, Raoof M, Wilson L, Wasif N. Comparison of rates and outcomes of readmission to index vs nonindex hospitals after major cancer surgery. JAMA Surg. 2018;153(8):719–27.
Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–90.
Song Y, Shannon AB, Concors SJ, et al. Are volume pledge standards worth the travel burden for major abdominal cancer operations? Ann Surg. 2022;275:e743–51.
Austin PC. Variance estimation when using inverse probability of treatment weighting (IPTW) with survival analysis. Stat Med. 2016;35(30):5642–55.
Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–79.
Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol. 2011;173(7):761–7.
Ali MS, Prieto-Alhambra D, Lopes LC, et al. Propensity score methods in health technology assessment: principles, extended applications, and recent advances. Front Pharmacol. 2019;10:973.
Yu Q, Wu X, Li B, Scribner RA. Multiple mediation analysis with survival outcomes: With an application to explore racial disparity in breast cancer survival. Stat Med. 2019;38(3):398–412.
MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci. 2000;1(4):173–81.
Urbach DR. Pledging to eliminate low-volume surgery. N Engl J Med. 2015;373(15):1388–90.
Fong ZV, Loehrer AP, Fernández-Del Castillo C, et al. Potential impact of a volume pledge on spatial access: a population-level analysis of patients undergoing pancreatectomy. Surgery. 2017;162(2):203–10.
Finlayson SR, Birkmeyer JD, Tosteson AN, Nease RF Jr. Patient preferences for location of care: implications for regionalization. Med Care. 1999;37(2):204–9.
Macedo FIB, Jayanthi P, Mowzoon M, Yakoub D, Dudeja V, Merchant N. The impact of surgeon volume on outcomes after pancreaticoduodenectomy: a meta-analysis. J Gastrointest Surg. 2017;21(10):1723–31.
Sheetz KH, Nuliyalu U, Nathan H, Sonnenday CJ. Association of surgeon case numbers of pancreaticoduodenectomies vs related procedures with patient outcomes to inform volume-based credentialing. JAMA Netw Open. 2020;3(4):e203850.
de Geus SWL, Hachey KJ, Nudel JD, et al. Volume of pancreas-adjacent operations favorably influences pancreaticoduodenectomy outcomes at lower volume pancreas centers. Ann Surg. 2022;276:e102–7.
Acher AW, Weber SM, Pawlik TM. Does the volume–outcome association in pancreas cancer surgery justify regionalization of care? A review of current controversies. Ann Surg Oncol. 2022;29:1257–68.
Funding
Research reported herein was supported in part through funding from the John Wayne Cancer Foundation (Costa Mesa, CA, USA).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Disclaimer: The results and opinions expressed in this article are those of the authors, and do not reflect the official policy or position of the US Army Medical Department, Department of the Army, Department of Defense, or the US Government.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kemp Bohan, P.M., Chang, SC., Grunkemeier, G.L. et al. Impact of Mediating and Confounding Variables on the Volume–Outcome Association in the Treatment of Pancreatic Cancer. Ann Surg Oncol 30, 1436–1448 (2023). https://doi.org/10.1245/s10434-022-12908-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12908-z