Abstract
Background
Nanoliposomal irinotecan (nal-IRI) is a promising novel hyperthermic intraperitoneal chemotherapy (HIPEC) agent given its enhanced efficacy against gastrointestinal tumors, safety profile, thermo-synergy, and heat stability. This report describes the first in-human phase 1 clinical trial of nal-IRI during cytoreductive surgery (CRS) and HIPEC.
Methods
Patients with peritoneal surface disease (PSD) from appendiceal and colorectal neoplasms were enrolled in a 3 + 3 dose-escalation trial using nal-IRI (70–280 mg/m2) during HIPEC for 30 min at 41 ± 1 °C. The primary outcome was safety. The secondary outcomes were pharmacokinetics (PK) and disease-free survival. Adverse events (AEs) categorized as grade 2 or higher were recorded. The serious AEs (SAEs) were mortality, grade ≥ 3 AEs, and dose-limiting toxicity (DLT). Irinotecan and active metabolite SN38 were measured in plasma and peritoneal washings.
Results
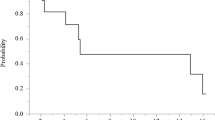
The study enrolled 18 patients, who received nal-IRI during HIPEC at 70 mg/m2 (n = 3), 140 mg/m2 (n = 6), 210 mg/m2 (n = 3), and 280 mg/m2 (n = 6). No DLT or mortality occurred. The overall morbidity for CRS/HIPEC was 39% (n = 7). Although one patient experienced neutropenia, no AE (n = 131) or SAE (n = 3) was definitively attributable to nal-IRI. At 280 mg/m2, plasma irinotecan and SN38 measurements showed maximum concentrations of 0.4 ± 0.6 µg/mL and 3.0 ± 2.4 ng/mL, a median time to maximum concentration of 24.5 and 26 h, and areas under the curve of 22.6 h*µg/mL and 168 h*ng/mL, respectively. At the 6-month follow-up visit, 83% (n = 15) of the patients remained disease-free.
Conclusions
In this phase 1 HIPEC trial (NCT04088786), nal-IRI was observed to be safe, and PK profiling showed low systemic absorption overall. These data support future studies testing the efficacy of nal-IRI in CRS/HIPEC.



Similar content being viewed by others
References
Fujimoto S, Shrestha RD, Kokubun M, et al. Clinical trial with surgery and intraperitoneal hyperthermic perfusion for peritoneal recurrence of gastrointestinal cancer. Cancer. 1989;64:154–60. https://doi.org/10.1002/1097-0142(19890701)64:1%3c154::aid-cncr2820640126%3e3.0.co;2-8.
The Chicago Consensus on peritoneal surface malignancies. management of appendiceal neoplasms. Cancer. 2020;126:2525–33. https://doi.org/10.1002/cncr.32881.
The Chicago Consensus on peritoneal surface malignancies. management of colorectal metastases. Cancer. 2020;126:2534–40. https://doi.org/10.1002/cncr.32874.
de Bree E, Michelakis D, Stamatiou D, Romanos J, Zoras O. Pharmacological principles of intraperitoneal and bidirectional chemotherapy. Pleura Peritoneum. 2017;2:47–62. https://doi.org/10.1515/pp-2017-0010.
Tournigand C, André T, Achille E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study. J Clin Oncol. 2004;22:229–37. https://doi.org/10.1200/jco.2004.05.113.
Rothenberg ML, Oza AM, Bigelow RH, et al. Superiority of oxaliplatin and fluorouracil-leucovorin compared with either therapy alone in patients with progressive colorectal cancer after irinotecan and fluorouracil-leucovorin: interim results of a phase III trial. J Clin Oncol. 2003;21:2059–69. https://doi.org/10.1200/jco.2003.11.126.
Chabot GG. Clinical pharmacokinetics of irinotecan. Clin Pharmacokinet. 1997;33:245–59. https://doi.org/10.2165/00003088-199733040-00001.
Elias D, Raynard B, Bonnay M, Pocard M. Heated intra-operative intraperitoneal oxaliplatin alone and in combination with intraperitoneal irinotecan: pharmacologic studies. Eur J Surg Oncol. 2006;32:607–13. https://doi.org/10.1016/j.ejso.2006.03.004.
Elias D, Goere D, Blot F, et al. Optimization of hyperthermic intraperitoneal chemotherapy with oxaliplatin plus irinotecan at 43 degrees C after compete cytoreductive surgery: mortality and morbidity in 106 consecutive patients. Ann Surg Oncol. 2007;14:1818–24. https://doi.org/10.1245/s10434-007-9348-1.
Elias D, Matsuhisa T, Sideris L, et al. Heated intraoperative intraperitoneal oxaliplatin plus irinotecan after complete resection of peritoneal carcinomatosis: pharmacokinetics, tissue distribution and tolerance. Ann Oncol. 2004;15:1558–65. https://doi.org/10.1093/annonc/mdh398.
Glockzin G, Gerken M, Lang SA, Klinkhammer-Schalke M, Piso P, Schlitt HJ. Oxaliplatin-based versus irinotecan-based hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with peritoneal metastasis from appendiceal and colorectal cancer: a retrospective analysis. BMC Cancer. 2014;14:807. https://doi.org/10.1186/1471-2407-14-807.
Wang-Gillam A, Li CP, Bodoky G, et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet. 2016;387:545–57. https://doi.org/10.1016/s0140-6736(15)00986-1.
FDA. Highlights of Prescribing Information, ONYVIDE (liposomal irinotecan) for injection. Drugs@FDA. 2015.
Wöll E, Thaler J, Keil F, et al. Oxaliplatin/irinotecan/bevacizumab followed by docetaxel/bevacizumab in inoperable locally advanced or metastatic gastric cancer patients–AGMT_GASTRIC-3. Anticancer Res. 2017;37:5553–8. https://doi.org/10.21873/anticanres.11987.
Adiwijaya BS, Kim J, Lang I, et al. Population pharmacokinetics of liposomal irinotecan in patients with cancer. Clin Pharmacol Ther. 2017;102:997–1005. https://doi.org/10.1002/cpt.720.
Kalra AV, Kim J, Klinz SG, et al. Preclinical activity of nanoliposomal irinotecan is governed by tumor deposition and intratumor prodrug conversion. Cancer Res. 2014;74:7003–13. https://doi.org/10.1158/0008-5472.Can-14-0572.
Frøysnes IS, Andersson Y, Larsen SG, et al. Novel treatment with intraperitoneal MOC31PE immunotoxin in colorectal peritoneal metastasis: results from the ImmunoPeCa phase 1 trial. Ann Surg Oncol. 2017;24:1916–22. https://doi.org/10.1245/s10434-017-5814-6.
Leonard SC, Lee H, Gaddy DF, et al. Extended topoisomerase 1 inhibition through liposomal irinotecan results in improved efficacy over topotecan and irinotecan in models of small cell lung cancer. Anticancer Drugs. 2017;28:1086–96. https://doi.org/10.1097/cad.0000000000000545.
Santos A, Zanetta S, Cresteil T, et al. Metabolism of irinotecan (CPT-11) by CYP3A4 and CYP3A5 in humans. Clin Cancer Res. 2000;6:2012–20.
Tossey JC, Reardon J, VanDeusen JB, Noonan AM, Porter K, Arango MJ. Comparison of conventional versus liposomal irinotecan in combination with fluorouracil for advanced pancreatic cancer: a single-institution experience. Med Oncol. 2019;36:87. https://doi.org/10.1007/s12032-019-1309-6.
Alavi S, Haeri A, Mahlooji I, Dadashzadeh S. Tuning the physicochemical characteristics of particle-based carriers for intraperitoneal local chemotherapy. Pharm Res. 2020;37:119. https://doi.org/10.1007/s11095-020-02818-8.
FDA. Highlights of Prescribing Information, COMPTOSAR (Irinotecan) Injection. Drugs@FDA. 2014.
Drummond DC, Noble CO, Guo Z, Hong K, Park JW, Kirpotin DB. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006;66:3271–7. https://doi.org/10.1158/0008-5472.Can-05-4007.
Das M, Huang L. Liposomal nanostructures for drug delivery in gastrointestinal cancers. J Pharmacol Exp Ther. 2019;370:647–56. https://doi.org/10.1124/jpet.118.254797.
Bilbro NA, Hirst A, Paez A, et al. The IDEAL reporting guidelines: a Delphi consensus statement stage-specific recommendations for reporting the evaluation of surgical innovation. Ann Surg. 2021;273:82–5. https://doi.org/10.1097/sla.0000000000004180.
Cotte E, Passot G, Tod M, et al. Closed abdomen hyperthermic intraperitoneal chemotherapy with irinotecan and mitomycin C: a phase I study. Ann Surg Oncol. 2011;18:2599–603. https://doi.org/10.1245/s10434-011-1651-1.
Malgras B, Gayat E, Aoun O, et al. Impact of combination chemotherapy in peritoneal mesothelioma hyperthermic intraperitoneal chemotherapy (HIPEC): the RENAPE study. Ann Surg Oncol. 2018;25:3271–9. https://doi.org/10.1245/s10434-018-6631-2.
Quenet F, Goéré D, Mehta SS, et al. Results of two bi-institutional prospective studies using intraperitoneal oxaliplatin with or without irinotecan during HIPEC after cytoreductive surgery for colorectal carcinomatosis. Ann Surg. 2011;254:294–301. https://doi.org/10.1097/SLA.0b013e3182263933.
Benson AB, Venook AP, Al-Hawary MM, et al. Colon cancer, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329–59. https://doi.org/10.6004/jnccn.2021.0012.
LoRusso PM, Li J, Burger A, et al. Phase I Safety, pharmacokinetic, and pharmacodynamic study of the Poly(ADP-ribose) polymerase (PARP) inhibitor Veliparib (ABT-888) in combination with irinotecan in patients with advanced solid tumors. Clin Cancer Res. 2016;22:3227–37. https://doi.org/10.1158/1078-0432.Ccr-15-0652.
ClinicalTrials.gov. Identifier NCT01770353, MM-398 (Nanoliposomal Irinotecan, Nal-IRI) to Determine Tumor Drug Levels and to Evaluate the Feasibility of Ferumoxytol Magnetic Resonance Imaging to Measure Tumor Associated Macrophages and to Predict Patient Response to Treatment. Bethesda (MD): National Library of Medicine (US). Accessed Feb 2022, https://clinicaltrials.gov/ct2/show/NCT01770353.
US Census Bureau. QuickFacts. Updated April 2022. https://www.census.gov/quickfacts/fact/table/fayettecountykentucky,suffolkcountynewyork/RHI125220.
Dimou A, Syrigos KN, Saif MW. Is there a role for mitomycin C in metastatic colorectal cancer? Expert Opin Investig Drugs. 2010;19:723–35. https://doi.org/10.1517/13543784.2010.485191.
Levine EA, Votanopoulos KI, Shen P, et al. A multicenter randomized trial to evaluate hematologic toxicities after hyperthermic intraperitoneal chemotherapy with oxaliplatin or mitomycin in patients with appendiceal tumors. J Am Coll Surg. 2018;226:434–43. https://doi.org/10.1016/j.jamcollsurg.2017.12.027.
Narasimhan V, Tan S, Kong J, et al. Prognostic factors influencing survival in patients undergoing cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for isolated colorectal peritoneal metastases: a systematic review and meta-analysis. Colorectal Dis. 2020;22:1482–95. https://doi.org/10.1111/codi.15003.
Rosa F, Galiandro F, Ricci R, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for colorectal peritoneal metastases: analysis of short- and long-term outcomes. Langenbecks Arch Surg. 2021. https://doi.org/10.1007/s00423-021-02353-z.
Spiegelberg J, Neeff H, Holzner P, Runkel M, Fichtner-Feigl S, Glatz T. Comparison of hyperthermic intraperitoneal chemotherapy regimens for treatment of peritoneal-metastasized colorectal cancer. World J Gastrointest Oncol. 2020;12:903–17. https://doi.org/10.4251/wjgo.v12.i8.903.
Zhang X, Wu Q, Wei M, Deng X, Gu C, Wang Z. Oxaliplatin versus mitomycin C in HIPEC for peritoneal metastasis from colorectal cancer: a systematic review and meta-analysis of comparative studies. Int J Colorect Dis. 2020;35:1831–9. https://doi.org/10.1007/s00384-020-03702-y.
Lee SJ, Jeon Y, Lee HW, Kang J, Baik SH, Park EJ. Impact of mitomycin C-induced neutropenia after hyperthermic intraperitoneal chemotherapy with cytoreductive surgery in colorectal cancer patients with peritoneal carcinomatosis. Ann Surg Oncol. 2022;29:2077–86. https://doi.org/10.1245/s10434-021-10924-z.
Bhatt A, de Hingh I, Van Der Speeten K, et al. HIPEC methodology and regimens: the need for an expert consensus. Ann Surg Oncol. 2021;28:9098–113. https://doi.org/10.1245/s10434-021-10193-w.
Acknowledgment
Ipsen provided financial support for this clinical trial. Ipsen had no input into the study design, analysis, or interpretation of results. Many thanks are extended to Donna Gilbreath for assistance with the associated visual abstract for this publication and the additional clinical research staff at UK (Shelley Cooper, BA; Heather Pavlik, RN) and SBUH (Margaret Andrew, RN; Giuseppina Caravella, MS, MPH; Sumbul Yousafi, MPH). Ipsen reviewed this manuscript for scientific accuracy but did not have any input into its content or its final approval.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Jinha M. Park is President, co-founder, and board member, and holds stock in The Radiology Experts, Inc. This research was supported by NIH training grant T32CA160003 (Megan M. Harper). The remaining authors have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Choi, M., Harper, M.M., Pandalai, P.K. et al. A Multicenter Phase 1 Trial Evaluating Nanoliposomal Irinotecan for Heated Intraperitoneal Chemotherapy Combined with Cytoreductive Surgery for Patients with Peritoneal Surface Disease. Ann Surg Oncol 30, 804–813 (2023). https://doi.org/10.1245/s10434-022-12723-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12723-6