Abstract
Background
Adding pembrolizumab to preoperative chemotherapy improves event-free survival in patients with early-stage triple-negative breast cancer (TNBC). However, owing to potential toxicities, the risk–benefit ratio of pembrolizumab must be considered. There is consensus that the addition of immunotherapy should be recommended in node-positive patients. This study is undertaken to determine nodal positivity rates in patients with TNBC presenting with cT1–2N0 disease undergoing upfront surgery and to evaluate the utility of axillary ultrasound and biopsy in the setting of a negative clinical examination.
Patients and Methods
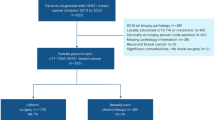
Patients with cT1–2N0 TNBC undergoing upfront surgery were identified from our institutional database (January 2016–February 2021; n = 343) and from the National Cancer Database (NCDB) (n = 46,015). Pathologic nodal status was determined. A second cohort of patients with cT1–T2 TNBC with a negative clinical examination was defined in our institutional database (n = 499), and utilization of axillary ultrasound was examined.
Results
For patients undergoing upfront surgery, pathologically positive nodes were found in 14.6% patients of our institutional cohort: 9.4% cT1a/b, 14.9% cT1c, and 20.8% cT2 tumors. In the NCDB cohort, 13.7% patients were node positive: 4.9% cT1a/b, 11.4% cT1c, and 19.7% cT2 tumors. For patients with a normal clinical examination undergoing axillary ultrasound, 7.5% of cT1c and 8.7% of cT2 had suspicious nodes biopsied and confirmed positive for metastasis.
Conclusions
Pathologic node-positive disease is found in > 10 and 20% patients with cT1cN0 and cT2N0 TNBC, respectively. Axillary ultrasound can be used to identify patients presenting with a normal clinical examination for whom preoperative pembrolizumab should be considered.
Similar content being viewed by others
References
Cortes J, Cescon DW, Rugo HS, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet. 2020;396:1817–28.
Schmid P, Cortes J, Pusztai L, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382:810–21.
Schmid P, Cortes J, Dent R, et al. KEYNOTE-522: phase 3 study of neoadjuvant pembrolizumab + chemotherapy versus placebo + chemotherapy, followed by adjuvant pembrolizumab versus placebo for early -stage triple-negative breast cancer. ESMO Virtual Plenary Session. 2021
Schmid P, Cortes J, Dent R, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386:556–67.
Schmid PC, J Dent, R Pusztai, L McArthur, H Kümmel, S Bergh, J Denkert, C Hee Park, Y Hui, R Harbeck, N Takahashi, M Untch, M Fasching, PA Cardoso, F Andersen, J Patt, D Danso, M Ferreira, M Mouret-Reynier, M-A Im, S-A Ahn, J-H Gion, M Baron-Hay, S Boileau, J-F Zhu, Y Pan, W Tryfonidis, K Karantza, O’Shaughnessy J. KEYNOTE-522 study of neoadjuvant pembrolizumab + chemotherapy vs placebo + chemotherapy, followed by adjuvant pembrolizumab vs placebo for early-stage TNBC: event-free survival sensitivity and subgroup analyses. San Antonio Breast Cancer Symposium.
Allison KH, Hammond MEH, Dowsett M, et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP guideline update. J Clin Oncol. 2020;38:1346–66.
Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Clin Oncol. 2010;28:2784–95.
Wolff AC, Hammond MEH, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. J Clin Oncol. 2018;36:2105–22.
Weiss A, King C, Grossmith S, et al. How often does retrieval of a clipped lymph node change adjuvant therapy recommendations? A prospective, consecutive, patient cohort study. Ann Surg Oncol. 2022;29:3764–71. https://doi.org/10.1245/s10434-022-11324-7.
NCCN Clinical Practice Guidelines in Oncology. Breast Cancer. 2022
Pamilo M, Soiva M, Lavast EM. Real-time ultrasound, axillary mammography, and clinical examination in the detection of axillary lymph node metastases in breast cancer patients. J Ultrasound Med. 1989;8:115–20.
Sacre RA. Clinical evaluation of axillar lymph nodes compared to surgical and pathological findings. Eur J Surg Oncol. 1986;12:169–73.
de Freitas R, Costa MV, Schneider SV, et al. Accuracy of ultrasound and clinical examination in the diagnosis of axillary lymph node metastases in breast cancer. Eur J Surg Oncol. 1991;17:240–4.
Krishnamurthy S, Sneige N, Bedi DG, et al. Role of ultrasound-guided fine-needle aspiration of indeterminate and suspicious axillary lymph nodes in the initial staging of breast carcinoma. Cancer. 2002;95:982–8.
Bedi DG, Krishnamurthy R, Krishnamurthy S, et al. Cortical morphologic features of axillary lymph nodes as a predictor of metastasis in breast cancer: in vitro sonographic study. AJR Am J Roentgenol. 2008;191:646–52.
Houssami N, Ciatto S, Turner RM, et al. Preoperative ultrasound-guided needle biopsy of axillary nodes in invasive breast cancer: meta-analysis of its accuracy and utility in staging the axilla. Ann Surg. 2011;254:243–51.
Acknowledgements
E.A.M. acknowledges support as the Rob and Karen Hale Distinguished Chair in Surgical Oncology. The authors also acknowledge Tonia Parker and Julie Vincuilla for their oversight of the Dana-Farber Brigham Cancer Center prospectively maintained breast clinical oncology quality database.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
E.A.M. reports compensated service on scientific advisory boards for AstraZeneca, Exact Sciences (formerly Genomic Health), Merck, Roche/Genentech; uncompensated service on steering committees for Bristol Myers Squibb, Lilly, and Roche/Genentech; and institutional research support from Roche/Genentech (via SU2C grant) and Gilead. A.W. reports institutional research support from Myriad. E.R. reports institutional research support from AstraZeneca. A.G.C. reports institutional research support from AstraZeneca, Gilead, and Merck. I.E.K. reports employment from AMAG Pharmaceuticals and Freeline Therapeutics; stock and other ownership interests from AMAG Pharmaceuticals, Freeline Therapeutics, and Vertex; honoraria from AstraZeneca, Roche/Genentech, and Celltrion; consulting or advisory role for AstraZeneca, Bristol Myers Squibb, Context Therapeutics, Daiichi Sankyo, Macrogenics, Merck, Novartis, Roche/Genentech, Seattle Genetics, and Taiho Pharmaceutical; and institutional research support from Roche/Genentech and Pfizer. N.U.L. reports stock and other ownership interests from Artera Inc.; consulting or advisory role for Affinia Therapeutics, Aleta Biopharma, AstraZeneca, Daichii-Sankyo, Denali Therapeutics, Olema Pharmaceuticals, Prelude Therapeutics, Puma, and Voyager Therapeutics; and institutional research support from AstraZeneca, Merck, Olema Pharmaceuticals, Roche/Genentech, Seattle Genetics, and Pfizer. E.P.W. reports honoraria from Exact Sciences (formerly Genomic Health) and Roche/Genentech; consulting or advisory role for Athenex, Carrick Therapeutics, G1 Therapeutics, GlaxoSmithKline, Gilead, Jounce Therapeutics, Leap Therapeutics, Lilly, Roche/Genentech, Syros Pharmaceuticals, and Zymeworks; and institutional research support from Roche/Genentech. S.M.T. reports consulting or advisory role for 4D Pharma, ARC Therapeutics, AstraZeneca, Athenex, BeyondSpring Pharmaceuticals, Blueprint Medicine, Bristol Myers Squibb, Certara, CytomX Therapeutics, Daiichi Sankyo, Eisai, Ellipses Pharma, G1 Therapeutics, Gillead, Kyowa Hakko Kirin, Lilly, Merck, Mersana, Nanostring Technologies, Nektar, Novartis, Odonate, OncoPep, OncoSec Medical, OncXerna, Paxman, Pfizer, Puma Biotechnology, Reveal Genomics, Roche/Genentech, Samsung Bioepis, Sanofi, Seattle Genetics, Silverback Therapeutics, Zentalis, and Zymeworks; and institutional research support from AstraZeneca, Bristol Myers Squibb, Cyclacel, Eisai, Exelixis, Gilead, Lily, Merck, Nanostring, Nektar, Novartis, Odonate Therapeutics, Roche/Genentech, Sanofi, Seattle Genetics, and Pfizer. T.A.K. reports speakers honoraria and compensated service on scientific Advisory Board of Exact Sciences (formerly Genomic Health); compensated service as faculty, PrecisCa cancer information service; and compensated service for a Global Advisory Board of Berins Healthcare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Mittendorf, E.A., Kantor, O., Weiss, A. et al. Nodal Positivity in Early-Stage Triple-Negative Breast Cancer: Implications for Preoperative Immunotherapy. Ann Surg Oncol 30, 100–106 (2023). https://doi.org/10.1245/s10434-022-12357-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-12357-8