Abstract
Background
Adding intraperitoneal paclitaxel (IP-PTX) to paclitaxel/5-fluoropyrimidine has shown promising results in patients with gastric cancer peritoneal metastases (GCPM) but has not been studied with standard-of-care platinum/fluoropyrimidine combinations. Our goal to was evaluate IP-PTX with capecitabine/oxaliplatin (XELOX) in GCPM.
Methods
Forty-four patients with GCPM received IP PTX (40 mg/m2, Days 1, 8), oral capecitabine (1000 mg/m2 twice daily, Days 1–14) and intravenous oxaliplatin (100 mg/m2, Day 1) in 21-day cycles. Patients with synchronous GCPM underwent conversion surgery if they had good response after chemotherapy, conversion to negative cytology, no extraperitoneal metastasis, and no peritoneal disease during surgery. The primary endpoint was overall survival and secondary endpoints were progression-free survival and safety. Outcomes from the trial were compared against a matched cohort of 39 GCPM patients who received systemic chemotherapy (SC) comprising platinum/fluoropyrimidine.
Results
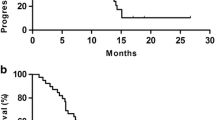
The median OS for the IP and SC groups was 14.6 and 10.6 months (hazard ratio [HR] 0.44; 95% confidence interval [CI], 0.26–0.74; p = 0.002). The median PFS for the IP and SC group was 9.5 and 4.4 months respectively (HR 0.39; 95% CI 0.25–0.66; p < 0.001). Patients in the SC group were younger (IP vs. SC, 61 vs. 56 years, p = 0.021) and had better performance status (ECOG 0, IP vs. SC, 47.7% vs. 76.9%, p = 0.007) compared with the IP cohort. In IP group, conversion surgery was performed in 36.1% (13/36) of patients, with a median OS of 24.2 (95% CI 13.1–35.3) months and 1-year OS of 84.6%.
Conclusions
IP PTX with XELOX is a promising treatment option for GCPM patients. In patients with good response, conversion surgery was feasible with favourable outcomes.


Similar content being viewed by others
References
Chu DZ, Lang NP, Thompson C, et al. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989;63(2):364–7. https://doi.org/10.1002/1097-0142(19890115)63:2%3c364::aid-cncr2820630228%3e3.0.co;2-v.
Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88(2):358–63. https://doi.org/10.1002/(sici)1097-0142(20000115)88:2%3c358::aid-cncr16%3e3.0.co;2-o.
Muro K, Van Cutsem E, Narita Y, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMO-ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2019;30(1):19–33. https://doi.org/10.1093/annonc/mdy502.
Dedrick RL, Myers CE, Bungay PM, et al. Pharmacokinetic rationale for peritoneal drug administration in the treatment of ovarian cancer. Cancer Treat Rep. 1978;62(1):1–11.
de Bree E, Michelakis D, Stamatiou D, et al. Pharmacological principles of intraperitoneal and bidirectional chemotherapy. Pleura Peritoneum. 2017;2(2):47–62. https://doi.org/10.1515/pp-2017-0010.
Chia DKA, So JBY. Recent advances in intra-peritoneal chemotherapy for gastric cancer. J Gastric Cancer. 2020;20(2):115–26. https://doi.org/10.5230/jgc.2020.20.e15.
Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med. 2006;354(1):34–43. https://doi.org/10.1056/NEJMoa052985.
Kobayashi D, Kodera Y. Intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2017;20(Suppl 1):111–21.
Ishigami H, Kitayama J, Kaisaki S, et al. Phase II study of weekly intravenous and intraperitoneal paclitaxel combined with S-1 for advanced gastric cancer with peritoneal metastasis. Ann Oncol Off J Eur Soc Med Oncol. 2010;21(1):67–70.
Fujiwara Y, Takiguchi S, Nakajima K, et al. Intraperitoneal docetaxel combined with S-1 for advanced gastric cancer with peritoneal dissemination. J Surg Oncol. 2012;105(1):38–42.
Imano M, Peng Y-F, Itoh T, et al. A preliminary study of single intraperitoneal administration of paclitaxel followed by sequential systemic chemotherapy with S-1 plus paclitaxel for advanced gastric cancer with peritoneal metastasis. Anticancer Res. 2012;32(9):4071–5.
Yamaguchi H, Kitayama J, Ishigami H, et al. A phase 2 trial of intravenous and intraperitoneal paclitaxel combined with S-1 for treatment of gastric cancer with macroscopic peritoneal metastasis. Cancer. 2013;119(18):3354–8.
Fushida S, Kinoshita J, Kaji M, et al. Phase I/II study of intraperitoneal docetaxel plus S-1 for the gastric cancer patients with peritoneal carcinomatosis. Cancer Chemother Pharmacol. 2013;71(5):1265–72.
Ishigami H, Yamaguchi H, Yamashita H, et al. Surgery after intraperitoneal and systemic chemotherapy for gastric cancer with peritoneal metastasis or positive peritoneal cytology findings. Gastric Cancer Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2017;20(Suppl 1):128–34.
Ishigami H, Fujiwara Y, Fukushima R, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol Off J Am Soc Clin Oncol. 2018;36(19):1922–9.
Yarema R, Ohorchak M, Hyrya P, et al. Gastric cancer with peritoneal metastases: efficiency of standard treatment methods. World J Gastrointest Oncol. 2020;12(5):569–81. https://doi.org/10.4251/wjgo.v12.i5.569.
Japanese Gastric Cancer Association. Japanese Classification of Gastric Carcinoma—2nd English Edition. Gastric Cancer. 1998;1(1):10-24.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
Laura HT, Jordan B, Philip B, et al. Protocol for the examination of specimens from patients with carcinoma of the stomach. In: Cancer protocol. College of American Pathologists; 2014.
Li Z, Xue K, Ying X, Ji J. PHOENIX-GC Trial: underpowered for significant results? J Clin Oncol. 2019;37(2):167. https://doi.org/10.1200/JCO.18.00364.
Maeda H, Kobayashi M, Sakamoto J. Evaluation and treatment of malignant ascites secondary to gastric cancer. World J Gastroenterol. 2015;21(39):10936–47. https://doi.org/10.3748/wjg.v21.i39.10936.
Ishigami H. Reply to Z. Li et al. J Clin Oncol. 2019;37(2):167–8. https://doi.org/10.1200/JCO.18.01003.
Lu Z, Zhang X, Liu W, et al. A multicenter, randomized trial comparing efficacy and safety of paclitaxel/capecitabine and cisplatin/capecitabine in advanced gastric cancer. Gastric Cancer. 2018;21(5):782–91. https://doi.org/10.1007/s10120-018-0809-y.
Jeung HC, Rha SY, Im CK, et al. A randomized phase 2 study of docetaxel and S-1 versus docetaxel and cisplatin in advanced gastric cancer with an evaluation of SPARC expression for personalized therapy. Cancer. 2011;117(10):2050–7. https://doi.org/10.1002/cncr.25729.
Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(suppl 5):v38-49. https://doi.org/10.1093/annonc/mdw350.
Kuo YC, Liu HT, Lin YL, et al. Modified biweekly oxaliplatin and capecitabine for advanced gastric cancer: a retrospective analysis from a medical center. Biomed J. 2014;37(3):141–6. https://doi.org/10.4103/2319-4170.117887.
Chao Y, Hsieh JS, Yeh HT, et al. A multicenter phase II study of biweekly capecitabine in combination with oxaliplatin as first-line chemotherapy in patients with locally advanced or metastatic gastric cancer. Cancer Chemother Pharmacol. 2014;73(4):799–806. https://doi.org/10.1007/s00280-014-2407-y.
Heger U, Bader F, Lordick F, et al. Interim endoscopy results during neoadjuvant therapy for gastric cancer correlate with histopathological response and prognosis. Gastric Cancer. 2014;17(3):478–88. https://doi.org/10.1007/s10120-013-0296-0.
Giganti F, Tang L, Baba H. Gastric cancer and imaging biomarkers: part 1—a critical review of DW-MRI and CE-MDCT findings. Eur Radiol. 2019;29(4):1743–53. https://doi.org/10.1007/s00330-018-5732-4.
Tang L, Wang XJ, Baba H, Giganti F. Gastric cancer and image-derived quantitative parameters: part 2—a critical review of DCE-MRI and 18F-FDG PET/CT findings. Eur Radiol. 2020;30(1):247–60. https://doi.org/10.1007/s00330-019-06370-x.
Emoto S, Ishigami H, Hidemura A, et al. Complications and management of an implanted intraperitoneal access port system for intraperitoneal chemotherapy for gastric cancer with peritoneal metastasis. Jpn J Clin Oncol. 2012;42(11):1013–9. https://doi.org/10.1093/jjco/hys129.
Acknowledgments
The authors thank the patients and their families who participated in this study, as well as colleagues from the Department of Surgery (National University Hospital and Ng Teng Fong General Hospital, Singapore), National University Cancer Institute, Singapore and Department of Pathology (National University Hospital, Singapore). This study received funding from the National Medical Research Council (NMRC) Translational and Clinical Flagship Programme (NMRC/TCR/009-NUHS/2013) and NMRC Centre Grants (NMRC/CG/012/2013 and NMRC/CG/M005/2017_NCIS). Dr. Raghav Sundar also is supported by a NMRC Fellowship (NMRC/Fellowship/0059/2018).
Funding
The National Medical Research Council (NMRC) oversees the development and advancement of medical research in Singapore. Trial registry: NCT01739894.
Author information
Authors and Affiliations
Contributions
The authors report no conflict of interests. Individual contributions to the submission are as follows: D.K.A.C. participated in data curation, formal analysis, investigation, methodology, supervision, validation, writing-original draft, writing-editing, and review of this submission. R.S. participated in formal analysis, investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. G.K. participated in conceptualizing, investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. J.J.A. participated in data curation, formal analysis, investigation, methodology, validation, writing-original draft, writing-editing, and review of this submission. J.H.Y.L. participated in formal analysis, investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. M.E.N. participated in formal analysis, investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. G.H.G. participated in formal analysis, investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. S.J.E. participated in formal analysis, investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. C.E.C. participated in conceptualizing, investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. H.L.T. participated in investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. J.H. participated in investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. N.Y.L.N. participated in investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. M.X.W.L. participated in investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. V.M. participated in investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. G.H.J.C. participated in investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. A.S.L.P. participated in investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. Y.L.E.A. participated in investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. J.R.E.C. participated in investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. J.S.J.L. participated in investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. J.L.T. participated in investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. A.L. participated in investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. Y.S. participated in investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. A.S. participated in investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. W.P.Y. participated in conceptualizing, data curation, funding acquisition, investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission. J.B.Y.S. participated in conceptualizing, data curation, funding acquisition, investigation, methodology, project administration, supervision, validation, writing-editing, and review of this submission.
Corresponding author
Ethics declarations
Disclosures
Sundar Raghav—Advisory board—Bristol Myers Squibb, Merck, Eisai, Bayer, Taiho, Novartis, MSD; Honoraria for talks—MSD, Eli Lilly, BMS, Roche, Taiho, Astra Zeneca, DKSH; Travel—Roche, Astra Zeneca, Taiho, Eisai; Research funding—Paxman Coolers, MSD.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chia, D.K.A., Sundar, R., Kim, G. et al. Outcomes of a Phase II Study of Intraperitoneal Paclitaxel plus Systemic Capecitabine and Oxaliplatin (XELOX) for Gastric Cancer with Peritoneal Metastases. Ann Surg Oncol 29, 8597–8605 (2022). https://doi.org/10.1245/s10434-022-11998-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11998-z