Abstract
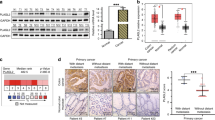
Colorectal cancer (CRC) is a major cause of cancer-related deaths. Metastasis is enhanced through epithelial-mesenchymal transition (EMT), a process primarily induced by the transforming growth factor beta (TGF-β)-mediated canonical Smad pathway. This study focused on plexin D1 (PLXND1), a chemoreceptor for the ligand SEMA3E to mechanosensory, showing that PLXND1 induces EMT via activation of the PI3K/AKT pathway in CRC cells. The findings showed that PLXND1-knockdown decreases cell migration and invasion significantly, and that the binding of p61-SEMA3E to the PLXND1 enhances the invasiveness and migration through EMT. Furin inhibitor suppresses EMT, decreasing cell migration and invasion. Furin cleaves full-length SEMA3E and converts it to p61-SEMA3E, suggesting that furin inhibitors block PLXND1 and p61-SEMA3E binding. Furin is a potential therapeutic target for the purpose of suppressing EMT by inhibiting the binding of p61-SEMA3E to PLXND1. In vivo experiments have shown that PLXND1-knockdown suppresses EMT. Mesenchymal cells labeled with ZEB1 showed heterogeneity depending on PLXND1 expression status. The high-expression group of PLXND1 in 182 CRC samples was significantly associated with poor overall survival compared with the low-expression group (P = 0.0352, median follow-up period of 60.7 months) using quantitative real-time polymerase chain reaction analysis. Further research is needed to determine whether cell fractions with a different expression of PLXND1 have different functions.





Similar content being viewed by others
References
Center MM, Jemal A, Smith RA, et al. Worldwide variations in colorectal cancer. CA Cancer J Clin. 2009;59:366–78.
Van der Pool AEM, Damhuis RA, Ijzermans JNM, et al. Trends in incidence, treatment, and survival of patients with stage IV colorectal cancer: a population-based series. Colorectal Dis. 2012;14:56–61.
Mantke R, Schmidt U, Wolff S, et al. Incidence of synchronous liver metastases in patients with colorectal cancer in relationship to clinico-pathologic characteristics: results of a German prospective multicentre observational study. Eur J Surg Oncol. 2012;38:259–65.
Skarkova V, Kralova V, Vitovcova B, et al. Selected aspects of chemoresistance mechanisms in colorectal carcinoma: a focus on epithelial-to-mesenchymal transition, autophagy, and apoptosis. Cells. 2019;8:234.
Thiery JP. Epithelial–mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–54.
Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial–mesenchymal transition. Nat Rev Mol Cell Biol. 2014;15:178–96.
Hao Y, Baker D, Ten Dijke P. TGF-β-mediated epithelial–mesenchymal transition and cancer metastasis. Int J Mol Sci. 2019;20:2767.
Francart ME, Lambert J, Vanwynsberghe AM, et al. Epithelial–mesenchymal plasticity and circulating tumor cells: travel companions to metastases. Dev Dyn. 2018;247:432–50.
Pastushenko I, Brisebarre A, Sifrim A, et al. Identification of the tumour transition states occurring during EMT. Nature. 2018;556:463–8.
Ieta K, Tanaka F, Haraguchi N, et al. Biological and genetic characteristics of tumor initiating cells in colon cancer. Ann Surg Oncol. 2008;15:638–48.
Luchino J, Hocine M, Amoureux MC, et al. Semaphorin 3E suppresses tumor cell death triggered by the plexin D1 dependence receptor in metastatic breast cancers. Cancer Cell. 2013;24:673–85.
Casazza A, Finisguerra V, Capparuccia L, et al. Sema3E-plexin D1-signaling drives human cancer cell invasiveness and metastatic spreading in mice. J Clin Investig. 2010;120:2684–98.
Tseng CH, Murray KD, Jou MF, et al. Sema3E/plexin-D1-mediated epithelial-to-mesenchymal transition in ovarian endometrioid cancer. PLoS ONE. 2011;6:e19396.
Casazza A, Kigel B, Maione F, et al. Tumor growth inhibition and anti-metastatic activity of a mutated furin-resistant semaphorin 3E isoform. EMBO Mol Med. 2012;4:234–50.
Neufeld G, Kessler O. The semaphorins: versatile regulators of tumour progression and tumour angiogenesis. Nat Rev Cancer. 2008;8:632–45.
Roodink I, Verrijp K, Raats J, et al. Plexin d1 is ubiquitously expressed on tumor vessels and tumor cells in solid malignancies. BMC Cancer. 2009;9:297.
Huang HY, Cheng YY, Liao WC, et al. SOX4 transcriptionally regulates multiple SEMA3/plexin family members and promotes tumor growth in pancreatic cancer. PLoS ONE. 2012;7:e48637.
Rehman M, Gurrapu S, Cagnoni G, et al. PlexinD1 is a novel transcriptional target and effector of notch-signaling in cancer cells. PLoS ONE. 2016;11:e0164660.
Jaaks P, Bernasconi M. The proprotein convertase furin in tumour progression: the PC furin in tumour progression. Int J Cancer. 2017;141:654–63.
Engle SJ, Hoying JB, Boivin GP, et al. Transforming growth factor β1 suppresses nonmetastatic colon cancer at an early stage of tumorigenesis. Cancer Res. 1999;59:3379–86.
Tang B, Vu M, Booker T, et al. TGF-β switches from tumor suppressor to prometastatic factor in a model of breast cancer progression. J Clin Investig. 2003;112:1116–24.
Guo X, Wang XF. Signaling cross-talk between TGF-beta/BMP and other pathways. Cell Res. 2009;19:71–88.
Javelaud D, Mauviel A. Crosstalk mechanisms between the mitogen-activated protein kinase pathways and Smad-signaling downstream of TGF-β implications for carcinogenesis. Oncogene. 2005;24:5742–50.
Braun E, Sauter D. Furin-mediated protein processing in infectious diseases and cancer. Clin Transl Immunol. 2019;8:e1073.
Oh J, Barve M, Matthews CM, et al. Phase II study of Vigil DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer. Gynecol Oncol. 2016;143:504–10.
Mehta V, Pang KL, Rozbesky D, et al. The guidance receptor PlexinD1 moonlights as endothelial mechanosensor. Nature. 2020;578:290–5.
Conti I, Rollins BJ. CCL2 (monocyte chemoattractant protein-1) and cancer. Semin Cancer Biol. 2004;14:149–54.
Qian BZ, Li J, Zhang H, et al. CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis. Nature. 2011;475:222–5.
Schlesinger M, Bendas G. Vascular cell adhesion molecule-1 (VCAM-1)—an increasing insight into its role in tumorigenicity and metastasis. Int J Cancer. 2015;136:2504–14.
Acknowledgment
We thank Ms. Miki Sakaue for her technical support in this study. This work was supported by a Grant-in-Aid for Young Scientists (19K18117). The funding source played no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hagihara, K., Haraguchi, N., Nishimura, J. et al. PLXND1/SEMA3E Promotes Epithelial–Mesenchymal Transition Partly via the PI3K/AKT-Signaling Pathway and Induces Heterogenity in Colorectal Cancer. Ann Surg Oncol 29, 7435–7445 (2022). https://doi.org/10.1245/s10434-022-11945-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11945-y