Abstract
Background
The effects of specific body mass index (BMI) category and sarcopenia within each BMI category on outcomes in patients undergoing esophageal surgery with esophageal squamous cell carcinoma have not been thoroughly examined.
Methods
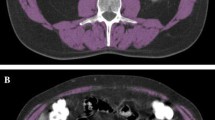
This study included 1141 patients. Sarcopenia was determined with a total psoas muscle cross-sectional area at the level of the third lumbar vertebra in computed tomography. The outcomes were long-term survival, including overall survival (OS) and recurrence-free survival (RFS), and postoperative complications.
Results
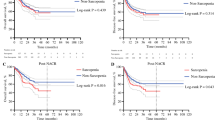
The overweight and no sarcopenia group was considered as the reference. After adjusting covariates, the underweight and the normal weight and sarcopenia groups both showed worse OS (underweight group: hazard ratio [HR] 2.04, 95% confidence interval [CI] 1.33–3.13, p = 0.001; normal weight and sarcopenia group: HR 1.93, 95% CI 1.39–2.69, p < 0.001) and worse RFS (underweight group: HR 1.78, 95% CI 1.19–2.67, p = 0.005; normal weight and sarcopenia group: HR 1.70, 95% CI 1.25–2.30, p = 0.001). In addition, the underweight group (odds ratio [OR] 4.74, 95% CI 2.05–10.96, p < 0.001), the normal weight and sarcopenia group (OR 3.26, 95% CI 1.60–6.62, p = 0.001), the overweight and sarcopenia group (OR 2.54, 95% CI 1.14–5.68, p = 0.023), and the obese and no sarcopenia group (OR 2.44, 95% CI 1.14–5.22, p = 0.021) were at significantly higher risk of postoperative 30-day composite complications.
Conclusions
Compared with the overweight and no sarcopenia group, the underweight and the normal weight and sarcopenia groups were associated with worse short- and long-term outcomes.




Similar content being viewed by others
References
Gertler R, Stein HJ, Langer R, et al. Long-term outcome of 2920 patients with cancers of the esophagus and esophagogastric junction: Evaluation of the New Union Internationale Contre le Cancer/American Joint Cancer Committee staging system. Ann Surg. 2011;253(4):689–98.
Baracos VE, Arribas L. Sarcopenic obesity: Hidden muscle wasting and its impact for survival and complications of cancer therapy. Ann Oncol. 2018;29:ii1–9.
Miao L, Chen H, Xiang J, Zhang Y. A high body mass index in esophageal cancer patients is not associated with adverse outcomes following esophagectomy. J Cancer Res Clin Oncol. 2015;141(5):941–50.
Zhang SS, Yang H, Luo KJ, et al. The impact of body mass index on complication and survival in resected oesophageal cancer: a clinical-based cohort and meta-analysis. Br J Cancer. 2013;109(11):2894–903.
Ji W, Zheng W, Li B, Cao C, Mao W. Influence of body mass index on the long-term outcomes of patients with esophageal squamous cell carcinoma who underwent esophagectomy as a primary treatment: a 10-year medical experience. Medicine (Baltimore). 2016;95(29):e4204.
Hasegawa T, Kubo N, Ohira M, et al. Impact of body mass index on surgical outcomes after esophagectomy for patients with esophageal squamous cell carcinoma. J Gastrointest Surg. 2015;19(2):226–33.
Wang SM, Fan JH, Jia MM, et al. Body mass index and long-term risk of death from esophageal squamous cell carcinoma in a Chinese population. Thorac Cancer. 2016;7(4):387–92.
Duan XF, Tang P, Shang XB, Jiang HJ, Zhao Q, Yu ZT. High Body Mass Index worsens survival in patients with esophageal squamous cell carcinoma after esophagectomy. Dig Surg. 2017;34(4):319–27.
Watanabe M, Ishimoto T, Baba Y, et al. Prognostic impact of body mass index in patients with squamous cell carcinoma of the esophagus. Ann Surg Oncol. 2013;20(12):3984–91.
Nakashima Y, Saeki H, Nakanishi R, et al. Assessment of sarcopenia as a predictor of poor outcomes after esophagectomy in elderly patients with esophageal cancer. Ann Surg. 2018;267(6):1100–4.
Huang CH, Lue KH, Hsieh TC, Liu SH, Wang TF, Peng TC. Association between sarcopenia and clinical outcomes in patients with esophageal cancer under neoadjuvant therapy. Anticancer Res. 2020;40(2):1175–81.
Oguma J, Ozawa S, Kazuno A, Yamamoto M, Ninomiya Y, Yatabe K. Prognostic significance of sarcopenia in patients undergoing esophagectomy for superficial esophageal squamous cell carcinoma. Dis Esophagus. 2019;32(7):doy104.
Paireder M, Asari R, Kristo I, et al. Impact of sarcopenia on outcome in patients with esophageal resection following neoadjuvant chemotherapy for esophageal cancer. Eur J Surg Oncol. 2017;43(2):478–84.
Jun IJ, Jo JY, Kim JI, et al. Impact of anesthetic agents on overall and recurrence-free survival in patients undergoing esophageal cancer surgery: a retrospective observational study. Sci Rep. 2017;7(1):14020.
von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Epidemiology. 2007;18(6):800–4.
Okumura S, Kaido T, Hamaguchi Y, et al. Visceral Adiposity and Sarcopenic Visceral Obesity are Associated with Poor Prognosis After Resection of Pancreatic Cancer. Ann Surg Oncol. 2017;24(12):3732–40.
Okamura H, Kimura N, Tanno K, et al. The impact of preoperative sarcopenia, defined based on psoas muscle area, on long-term outcomes of heart valve surgery. J Thorac Cardiovasc Surg. 2019;157(3):1071-1079 e1073.
Kurumisawa S, Kawahito K. The psoas muscle index as a predictor of long-term survival after cardiac surgery for hemodialysis-dependent patients. J Artif Organs. 2019;22(3):214–21.
Jones KI, Doleman B, Scott S, Lund JN, Williams JP. Simple psoas cross-sectional area measurement is a quick and easy method to assess sarcopenia and predicts major surgical complications. Colorectal Dis. 2015;17(1):O20-26.
Seo MH, Lee WY, Kim SS, et al. 2018 Korean Society for the Study of Obesity guideline for the management of obesity in Korea. J Obes Metab Syndr. 2019;28(1):40–5.
Jammer I, Wickboldt N, Sander M, et al. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: European Perioperative Clinical Outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol. 2015;32(2):88–105.
Lucas DJ, Pawlik TM. Quality improvement in gastrointestinal surgical oncology with American College of Surgeons National Surgical Quality Improvement Program. Surgery. 2014;155(4):593–601.
Schmidt HM, El Lakis MA, Markar SR, Hubka M, Low DE. Accelerated recovery within standardized recovery pathways after esophagectomy: a prospective cohort study assessing the effects of early discharge on outcomes, readmissions, patient satisfaction, and costs. Ann Thorac Surg. 2016;102(3):931–9.
Ozawa Y, Nakano T, Taniyama Y, et al. Evaluation of the impact of psoas muscle index, a parameter of sarcopenia, in patients with esophageal squamous cell carcinoma receiving neoadjuvant therapy. Esophagus. 2019;16(4):345–51.
Kobayashi A, Kaido T, Hamaguchi Y, et al. Impact of sarcopenic obesity on outcomes in patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg. 2019;269(5):924–31.
Prado CM, Lieffers JR, McCargar LJ, et al. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9(7):629–35.
Anandavadivelan P, Brismar TB, Nilsson M, Johar AM, Martin L. Sarcopenic obesity: a probable risk factor for dose limiting toxicity during neo-adjuvant chemotherapy in oesophageal cancer patients. Clin Nutr. 2016;35(3):724–30.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140(6):883–99.
Ronti T, Lupattelli G, Mannarino E. The endocrine function of adipose tissue: an update. Clin Endocrinol (Oxf). 2006;64(4):355–65.
Caulfield LE, de Onis M, Blössner M, Black RE. Undernutrition as an underlying cause of child deaths associated with diarrhea, pneumonia, malaria, and measles. Am J Clin Nutr. 2004;80(1):193–8.
Hickman D, Jones MK, Zhu S, et al. The effect of malnutrition on norovirus infection. mBio. 2014;5(2):e01032-e1013.
McGorrian C, Yusuf S, Islam S, et al. Estimating modifiable coronary heart disease risk in multiple regions of the world: the INTERHEART Modifiable Risk Score. Eur Heart J. 2011;32(5):581–9.
Wolf MJ, Adili A, Piotrowitz K, et al. Metabolic activation of intrahepatic CD8+ T cells and NKT cells causes nonalcoholic steatohepatitis and liver cancer via cross-talk with hepatocytes. Cancer Cell. 2014;26(4):549–64.
Meeuwsen S, Horgan GW, Elia M. The relationship between BMI and percent body fat, measured by bioelectrical impedance, in a large adult sample is curvilinear and influenced by age and sex. Clin Nutr. 2010;29(5):560–6.
Nelke C, Dziewas R, Minnerup J, Meuth SG, Ruck T. Skeletal muscle as potential central link between sarcopenia and immune senescence. EBioMedicine. 2019;49:381–8.
Templeton AJ, McNamara MG, Seruga B, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106(6):dju124.
Uretsky S, Messerli FH, Bangalore S, et al. Obesity paradox in patients with hypertension and coronary artery disease. Am J Med. 2007;120(10):863–70.
Le-Bert G, Santana O, Pineda AM, Zamora C, Lamas GA, Lamelas J. The obesity paradox in elderly obese patients undergoing coronary artery bypass surgery. Interact Cardiovasc Thorac Surg. 2011;13(2):124–7.
Kim BJ, Lee SH, Jung KH, et al. Dynamics of obesity paradox after stroke, related to time from onset, age, and causes of death. Neurology. 2012;79(9):856–63.
Vemmos K, Ntaios G, Spengos K, et al. Association between obesity and mortality after acute first-ever stroke: the obesity-stroke paradox. Stroke. 2011;42(1):30–6.
Hong NS, Kim KS, Lee IK, et al. The association between obesity and mortality in the elderly differs by serum concentrations of persistent organic pollutants: a possible explanation for the obesity paradox. Int J Obes (Lond). 2012;36(9):1170–5.
Muscaritoli M, Anker SD, Argilés J, et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics.” Clin Nutr. 2010;29(2):154–9.
Cruz-Jentoft AJ, Bahat G, Bauer J, et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48(1):16–31.
Baracos VE. Psoas as a sentinel muscle for sarcopenia: a flawed premise. J Cachexia Sarcopenia Muscle. 2017;8(4):527–8.
Funding
No sources of funding were used to assist in the preparation of this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, G.W., Nam, JS., Abidin, M.F.B.Z. et al. Impact of Body Mass Index and Sarcopenia on Short- and Long-Term Outcomes After Esophageal Cancer Surgery: An Observational Study. Ann Surg Oncol 29, 6871–6881 (2022). https://doi.org/10.1245/s10434-022-11944-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11944-z