Abstract
Introduction
Although the prognosis of patients with resected perihilar cholangiocarcinoma (PHC) with histological lymph node metastasis (LNM) is poor, preoperative prediction of LNM is difficult. This study aimed to evaluate the diagnostic performance of diffusion-weighted magnetic resonance imaging (DWI) for LNM of PHC.
Method
Consecutive patients who underwent surgical resection of PHC between January 2012 and May 2020 were retrospectively reviewed. The lymph node (LN) area (mm2) and apparent diffusion coefficient (ADC) value ( × 10−3 mm2/s) of pericholedochal LNs were measured by DWI. The characteristics of the patients and the LNs were evaluated according to the histological presence or absence of regional LNM. Univariate and multivariate analyses were performed to identify the predictors of LNM of PHC.
Results
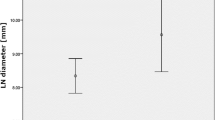
Of the 93 eligible patients, 49 (53%) were LNM positive and 44 (47%) were LNM negative. Although the characteristics of the patients were similar between the two groups, the mean ADC value was significantly lower in the LNM positive group than in the LNM negative group. On multivariate analysis, mean ADC value ≤1.80 × 10−3 mm2/s was independently associated with LNM of PHC (risk ratio: 12.5, 95% confidence interval: 3.05–51.4; p = 0.0004). The sensitivity, specificity and accuracy of mean ADC values ≤ 1.80 × 10−3 mm2/s for predicting LNM of PHC were 94%, 55% and 75%, respectively.
Conclusions
DWI might be useful for the preoperative diagnosis of LNM of PHC.


Similar content being viewed by others
References
Miyazaki M, Kato A, Ito H, et al. Combined vascular resection in operative resection for hilar cholangiocarcinoma: does it work or not? Surgery. 2007;141:581–8.
Shimizu H, Sawada S, Kimura F, et al. Clinical significance of biliary vascular anatomy of the right liver for hilar cholangiocarcinoma applied to left hemihepatectomy. Ann Surg. 2009;49:435–9.
Shimizu H, Kimura F, Yoshidome H, et al. Aggressive surgical resection for hilar cholangiocarcinoma of the left-side predominance. Ann Surg. 2010;251:281–6.
Hosokawa I, Shimizu H, Yoshidome H, et al. Surgical strategy for hilar cholangiocarcinoma of the left-side predominance: current role of left trisectionectomy. Ann Surg. 2014;259:1178–85.
Hosokawa I, Ohtsuka M, Yoshitomi H, et al. Right intersectional transection plane based on portal inflow in left trisectionectomy. Surg Radiol Anat. 2019;41:589–93.
Hosokawa I, Shimizu H, Ohtsuka M, et al. Liver transection-first approach in left trisectionectomy for perihilar cholangiocarcinoma. Ann Surg Oncol. 2020;27:2381–6.
Hosokawa I, Shimizu H. Left trisectionectomy for Bismuth-Corlette type IV perihilar cholangiocarcinoma of the left-sided predominance. Ann Surg Oncol. 2020;27:2387–8.
Aoba T, Ebata T, Yokoyama Y, et al. Assessment of nodal status for perihilar cholangiocarcinoma: location, number, or ratio of involved nodes. Ann Surg. 2013;257:718–25.
Nagino M. Perihilar cholangiocarcinoma: a surgeon’s viewpoint on current topics. J Gastroenterol. 2012;47:1165–76.
Rassam F, Roos E, van Lienden KP, et al. Modern work-up and extended resection in perihilar cholangiocarcinoma: the AMC experience. Langenbecks Arch Surg. 2018;403:289–307.
Hirata A, Hayano K, Ohira G, et al. Volumetric histogram analysis of apparent diffusion coefficient as a biomarker to predict survival of esophageal cancer patients. Ann Surg Oncol. 2020;27:3083–9.
Hayano K, Hirata A, Matsubara H. MRI-derived biomarker to select optimal treatment for esophageal cancer patients. Ann Surg Oncol. 2020;27:3090–1.
Hayano K, Miura F, Amano H, et al. Correlation of apparent diffusion coefficient measured by diffusion-weighted MRI and clinicopathological features in pancreatic cancer patients. J Hepatobiliary Pancreat Sci. 2013;20:243–8.
Hayano K, Miura F, Wada K, et al. Diffusion-weighted MR imaging of pancreatic cancer and inflammation: prognostic significance of pancreatic inflammation in pancreatic cancer patients. Pancreatology. 2016;16:121–6.
Shuto K, Kono T, Shiratori T, et al. Diagnostic performance of diffusion-weighted magnetic resonance imaging in assessing lymph node metastasis of esophageal cancer compared with PET. Esophagus. 2020;17:239–49.
Promsorn J, Soontrapa W, Somsap K, et al. Evaluation of diagnostic performance of apparent diffusion coefficient (ADC) values on diffusion-weighted magnetic resonance imaging (DWI) in differentiating between benign and metastatic lymph nodes in cases of cholangiocarcinoma. Abdom Radiol. 2019;44:473–81.
Holzapfel K, Gaa J, Schubert EC, et al. Value of diffusion-weighted MR imaging in the diagnosis of lymph node metastases in patients with cholangiocarcinoma. Abdom Radiol. 2016;41:1937–41.
Hosokawa I, Shimizu H, Yoshitomi H, et al. Impact of biliary drainage on multidetector-row computed tomography on R0 resection of perihilar cholangiocarcinoma. World J Surg. 2018;42:3676–84.
Hosokawa I, Shimizu H, Yoshitomi H, et al. Outcomes of left trisectionectomy and right hepatectomy for perihilar cholangiocarcinoma. HPB. 2019;21:489–98.
Hosokawa I, Furukawa K, Takayashiki T, et al. Surgical implications of the confluence patterns of the left intrahepatic bile ducts in right hepatectomy for perihilar cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2022;29:460–8.
Japanese Society of Hepato-Biliary-Pancreatic Surgery (JSHBPS). General rules for surgical and pathological studies on cancer of the biliary tract. 7th edn. Tokyo: Kanehara; 2021.
Kato A, Shimizu H, Ohtsuka M, et al. Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer: a retrospective single-center study. Ann Surg Oncol. 2013;20:318–24.
Kato A, Shimizu H, Ohtsuka M, et al. Downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer patients treated with Gemcitabine plus Cisplatin combination therapy followed by radical surgery. Ann Surg Oncol. 2015;22:1093–9.
Kiriyama S, Kozaka K, Takada T, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholangitis (with videos). J Hepatobiliary Pancreat Sci. 2018;25:17–30.
Mansour JC, Aloia TA, Crane CH, et al. Hilar cholangiocarcinoma: expert consensus statement. HPB. 2015;17:691–9.
Engels JT, Balfe DM, Lee JK. Biliary cholangiocarcinoma CT evaluation of extrahepatic spread. Radiology. 1989;172:35–40.
Lee HY, Kim SH, Lee JM, et al. Preoperative assessment of resectability of hepatic hilar cholangiocarcinoma: combined CT and cholangiography with revised criteria. Radiology. 2006;239:113–21.
Vilgrain V. Staging cholangiocarcinoma by imaging studies. HPB. 2008;10:106–9.
Dorfman RE, Alpern MB, Gross BH, et al. Upper abdominal lymph nodes: criteria for normal size determined with CT. Radiology. 1991;180:319–22.
Noji T, Kondo S, Hirano S, et al. Computed tomography evaluation of regional lymph node metastases in patients with biliary cancer. Br J Surg. 2008;95:92–6.
Kluge R, Schmidt F, Caca K, et al. Positron emission tomography with [18F] Fluoro-2-deoxy-d-glucose for diagnosis and staging of bile duct cancer. Hepatology. 2001;33:1029–35.
Ruys AT, Bennink RJ, van Westreenen HL, et al. FDG-positron emission tomography/computed tomography and standardized uptake value in the primary diagnosis and staging of hilar cholangiocarcinoma. HPB. 2011;13:256–62.
Malikowski T, Levy MJ, Gleeson FC, et al. Endoscopic ultrasound/fine needle aspiration is effective for lymph node staging in patients with cholangiocarcinoma. Hepatology. 2020;72:940–8.
Takahashi D, Mizuno T, Yokoyama Y, et al. Adjuvant S-1 vs gemcitabine for node-positive perihilar cholangiocarcinoma: a propensity score-adjusted analysis. J Hepatobiliary Pancreat Sci. 2021;28:716–26.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors declare that they have no conflicts of interest and received no funding support for this research.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hosokawa, I., Hayano, K., Furukawa, K. et al. Preoperative Diagnosis of Lymph Node Metastasis of Perihilar Cholangiocarcinoma Using Diffusion-Weighted Magnetic Resonance Imaging. Ann Surg Oncol 29, 5502–5510 (2022). https://doi.org/10.1245/s10434-022-11931-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11931-4