Abstract
Background
The SINODAR-ONE trial is a prospective noninferiority multicenter randomized study aimed at assessing the role of axillary lymph node dissection (ALND) in patients undergoing either breast-conserving surgery or mastectomy for T1–2 breast cancer (BC) and presenting one or two macrometastatic sentinel lymph nodes (SLNs). The endpoints were to evaluate whether SLN biopsy (SLNB) only was associated with worsening of the prognosis compared with ALND in terms of overall survival (OS) and relapse.
Methods
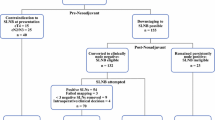
Patients were randomly assigned (1:1 ratio) to either removal of ≥ 10 axillary level I/II non-SLNs followed by adjuvant therapy (standard arm) or no further axillary treatment (experimental arm).
Results
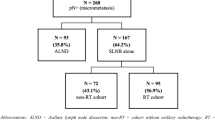
The trial started in April 2015 and ceased in April 2020, involving 889 patients. Median follow-up was 34.0 months. There were eight deaths (ALND, 4; SNLB only, 4), with 5-year cumulative mortality of 5.8% and 2.1% in the standard and experimental arm, respectively (p = 0.984). There were 26 recurrences (ALND 11; SNLB only, 15), with 5-year cumulative incidence of recurrence of 6.9% and 3.3% in the standard and experimental arm, respectively (p = 0.444). Only one axillary lymph node recurrence was observed in each arm. The 5-year OS rates were 98.9% and 98.8%, in the ALND and SNLB-only arm, respectively (p = 0.936).
Conclusions
The 3-year survival and relapse rates of T1–2 BC patients with one or two macrometastatic SLNs treated with SLNB only, and adjuvant therapy, were not inferior to those of patients treated with ALND. These results do not support the use of routine ALND.




Similar content being viewed by others
References
Gill G. Sentinel-lymph-node-based management or routine axillary clearance? One-year outcomes of sentinel node biopsy versus axillary clearance (SNAC): a randomized controlled surgical trial. Ann Surg Oncol. 2009. https://doi.org/10.1245/s10434-008-0229-z.
Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010. https://doi.org/10.1016/S1470-2045(10)70207-2.
Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial. J Natl Cancer Inst. 2006. https://doi.org/10.1093/jnci/djj158.
Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003. https://doi.org/10.1056/nejmoa012782.
Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group trial Z0011. J Clin Oncol. 2007. https://doi.org/10.1200/JCO.2006.07.4062.
Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: ten-year results: of a randomized controlled study. Ann Surg. 2010. https://doi.org/10.1097/SLA.0b013e3181c0e92a.
Canavese G, Bruzzi P, Catturich A, et al. Sentinel lymph node biopsy versus axillary dissection in node-negative early-stage breast cancer: 15-year follow-up update of a randomized clinical trial. Ann Surg Oncol. 2016. https://doi.org/10.1245/s10434-016-5177-4.
Bleyer A, Welch HG. Effect of Three decades of screening mammography on breast-cancer incidence. N Engl J Med. 2012. https://doi.org/10.1056/nejmoa1206809.
Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: a metaanalysis. Cancer. 2006. https://doi.org/10.1002/cncr.21568.
Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007. https://doi.org/10.1016/S1470-2045(07)70278-4.
Goyal A, Douglas-Jones A, Newcombe RG, Mansel RE. Predictors of non-sentinel lymph node metastasis in breast cancer patients. Eur J Cancer. 2004. https://doi.org/10.1016/j.ejca.2004.04.006.
Hwang RF, Gonzalez-Angulo AM, Yi M, et al. Low locoregional failure rates in selected breast cancer patients with tumor-positive sentinel lymph nodes who do not undergo completion axillary dissection. Cancer. 2007. https://doi.org/10.1002/cncr.22847.
Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009. https://doi.org/10.1200/JCO.2008.19.5750.
Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American college of surgeons oncology group z0011 randomized trial. Ann Surg. 2010. https://doi.org/10.1097/SLA.0b013e3181f08f32.
Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA J Am Med Assoc. 2017. https://doi.org/10.1001/jama.2017.11470.
Tinterri C, Canavese G, Bruzzi P, Dozin B. SINODAR ONE, an ongoing randomized clinical trial to assess the role of axillary surgery in breast cancer patients with one or two macrometastatic sentinel nodes. Breast. 2016. https://doi.org/10.1016/j.breast.2016.06.016.
Meinert CL. Clinical trials: design, conduct and analysis. Clin Trials Des Conduct Anal. 2009. https://doi.org/10.1093/acprof:oso/9780195035681.001.0001.
Piaggio G, Elbourne DR, Pocock SJ, Evans SJW, Altman DG. Reporting of noninferiority and equivalence randomized trials: extension of the CONSORT 2010 statement. JAMA - J Am Med Assoc. 2012. https://doi.org/10.1001/jama.2012.87802.
Fisher B, Jeong JH, Anderson S, Bryant J, Fisher ER, Wolmark N. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002. https://doi.org/10.1056/nejmoa020128.
Greco M, Agresti R, Cascinelli N, et al. Breast cancer patients treated without axillary surgery: clinical implications and biologic analysis. Ann Surg. 2000. https://doi.org/10.1097/00000658-200007000-00001.
Sekine C, Nakano S, Mibu A, Otsuka M, Oinuma T, Takeyama H. Breast cancer hormone receptor negativity, triple-negative type, mastectomy and not receiving adjuvant radiotherapy were associated with axillary recurrence after sentinel lymph node biopsy. Asian J Surg. 2020. https://doi.org/10.1016/j.asjsur.2019.05.001.
Güth U, Myrick ME, Viehl CT, Schmid SM, Obermann EC, Weber WP. The post ACOSOG Z0011 era: Does our new understanding of breast cancer really change clinical practice? Eur J Surg Oncol. 2012. https://doi.org/10.1016/j.ejso.2012.04.018.
Voutsadakis IA, Spadafora S. Recommendation for omitting axillary lymph node dissection should be individualized in patients with breast cancer with one or two positive sentinel lymph nodes. J Clin Oncol. 2014. https://doi.org/10.1200/JCO.2014.57.1190.
Goyal A, Dodwell D, Reed MW, Coleman RE. Axillary treatment in women with one or two sentinel nodes with macrometastases: more evidence is needed to inform practice. J Clin Oncol. 2014. https://doi.org/10.1200/JCO.2014.57.3717.
Dengel LT, Van Zee KJ, King TA, et al. Axillary dissection can be avoided in the majority of clinically node-negative patients undergoing breast-conserving therapy. Ann Surg Oncol. 2014. https://doi.org/10.1245/s10434-013-3200-6.
Caudle AS, Hunt KK, Kuerer HM, et al. Multidisciplinary considerations in the implementation of the findings from the American College of Surgeons Oncology Group (ACOSOG) Z0011 study: a practice-changing trial. Ann Surg Oncol. 2011. https://doi.org/10.1245/s10434-011-1593-7.
Tsao MW, Cornacchi SD, Hodgson N, et al. A population-based study of the effects of a regional guideline for completion axillary lymph node dissection on axillary surgery in patients with breast cancer. Ann Surg Oncol. 2016. https://doi.org/10.1245/s10434-016-5310-4.
Yao K, Liederbach E, Pesce C, Wang CH, Winchester DJ. Impact of the American College of Surgeons Oncology Group Z0011 randomized trial on the number of axillary nodes removed for patients with early-stage breast cancer. J Am Coll Surg. 2015. https://doi.org/10.1016/j.jamcollsurg.2015.02.035.
Morrow M, Jagsi R, Chandler M, Shumway D, Katz SJ. Surgeon attitudes toward the omission of axillary dissection in early breast cancer. JAMA Oncol. 2018. https://doi.org/10.1001/jamaoncol.2018.1908.
de Boniface J, Frisell J, Andersson Y, et al. Survival and axillary recurrence following sentinel node-positive breast cancer without completion axillary lymph node dissection: the randomized controlled SENOMAC trial. BMC Cancer. 2017. https://doi.org/10.1186/s12885-017-3361-y.
Goyal A, Dodwell D. POSNOC: a randomised trial looking at axillary treatment in women with one or two sentinel nodes with macrometastases. Clin Oncol. 2015. https://doi.org/10.1016/j.clon.2015.07.005.
Reimer T, Stachs A, Nekljudova V, et al. Restricted axillary staging in clinically and sonographically node-negative early invasive breast cancer (C/IT1-2) in the context of breast conserving therapy: first results following commencement of the intergroup-sentinel-mamma (INSEMA) trial. Geburtshilfe Frauenheilkd. 2017. https://doi.org/10.1055/s-0042-122853.
Garcia-Etienne CA, Ferrari A, Della Valle A, et al. Management of the axilla in patients with breast cancer and positive sentinel lymph node biopsy: an evidence-based update in a European breast center. Eur J Surg Oncol. 2019. https://doi.org/10.1016/j.ejso.2019.08.013.
Gondos A, Jansen L, Heil J, et al. Time trends in axilla management among early breast cancer patients: persisting major variation in clinical practice across European centers. Acta Oncol (Madr). 2016. https://doi.org/10.3109/0284186X.2015.1136751.
Acknowledgment
We thank Mrs Elena Bissolotti for clinical data acquisition and collection.
The SINODAR-ONE Collaborative Group: Olindo Custodero, MD8, Vito Leopoldo Troilo, MD8, Mario Taffurelli, MD9, Maria Cristina Cucchi, MD10, Valentina Galluzzo, MD10, Carlo Cabula, MD11, Roberta Cabula, MD11, Maria Grazia Lazzaretti, MD12, Francesco Caruso, MD, PhD13, Gaetano Castiglione, MD13, Simona Grossi, MD14, Maria Saveria Tavoletta, MD14, Camilla Rossi, MD15, Annalisa Curcio, MD, PhD16, Daniele Friedman, MD17, Piero Fregatti, MD, PhD17, Carla Magni, MD18, Giovanni Tazzioli, MD19, Simona Papi, MD19, Riccardo Giovanazzi, MD20, Camelia Chifu, MD20, Rossella Bettini, MD21, Modestino Pezzella, MD21, Silvia Michieletto, MD22, Tania Saibene, MD22, Manuela Roncella, MD23, Matteo Ghilli, MD23, Andrea Sibilio, MD24, Anna Cariello, MD24, Saverio Coiro, MD25, Giuseppe Falco, MD25, Emanuele Zarba Meli, MD26, Lucio Fortunato, MD26, Luigi Ciuffreda, MD27, Roberto Murgo, MD, PhD27, Claudio Battaglia, MD28, Luca Rubino, MD28, Nicoletta Biglia, MD, PhD29, Valentina Bounous, MD, PhD29, Francesca Angela Rovera, MD30, Corrado Chiappa, MD30, Giovanni Pollini, MD31, Sara Mirandola, MD31, Graziano Meneghini, MD32, Francesco Di Bartolo, MD32
8UOSVD Chirurgia Senologica – Breast Unit ASL BA P.O. San Paolo, Bari, Italy; 9IRCCS – Azienda Ospedaliero Universitaria di Bologna – Policlinico di Sant’Orsola, Bologna, Italy; 10U.O. Chirurgia Senologica Dipartimento Chirurgie Specialistiche USL di Bologna, Italy; 11 Oncologia e Senologia Azienda di Rilievo Nazionale Alta Specializzazione ARNAS – Cagliari, Italy; 12Unità semplice di Chirurgia Senologica, Ospedale Ramazzini di Carpi, AUSL Modena, Italy; 13Humanitas Istituto Clinico Catanese – Contrada Cubba SP 54 n. 11, 95045, Misterbianco (CT), Italy; 14U.O.C. di Chirurgia Generale a indirizzo Senologico – EUSOMA Breast Centre ASL 2 Abruzzo, Italy; 15U.O. Chirurgia Senologica, Ospedale degli Infermi, Viale Stradone 9, Faenza, Italy; 16Chirurgia Senologica Ospedale di Forlì – Ravenna, Azienda USL della Romagna, Italy; 17UO Clinica di Chirurgia Policlinico San Martino – IRCCS, Genova, Italy; 18SSD Breast Unit ASST Lecco, Italy; 19Chirurgia Oncologica Senologica, Azienda Ospedaliera-Universitaria di Modena, Italy; 20Breast Unit – Surgery – San Gerardo Hospital, Monza, Italy; 21UOC Chirurgia Senologica IRCCS Sacro Cuore Don Calabria, Presidio Ospedaliero Accreditato – Regione Veneto, Italy; 22Breast Surgery, Istituto Oncologico Veneto, Padova, Italy; 23Breast Surgery Unit, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy; 24Oncology Department AUSL Romagna, Ravenna Hospital, Ravenna, Italy; 25Breast Surgery Unit, Azienda USL – IRCCS di Reggio Emilia, Italy; 26UOC Senologia, Azienda ospedaliera San Giovanni-Addolorata, Rome, Italy; 27Chirurgia Senologica, Dipartimento Scienze Chirurgiche, IRCCS ‘Casa Sollievo della Sofferenza’ Opera di San Pio da Pietralcina, 71013, San Giovanni Rotondo (FG), Italy; 28Breast Unit, Civic Hospital of Sanremo, ASL 1 Imperiese, Sanremo, Italy; 29SCDU Gin e Ost, Ospedale Mauriziano Umberto primo, Torino, Italy; 30Centro Ricerche in Senologia, Università degli Studi dell’Insubria, S.S.D. Breast Unit, ASST SetteLaghi, Varese, Italy; 31UOC Chirurgia Senologica – Breast Unit, AOUI Verona, Italy; 32Breast Unit, Ospedale Montecchio Maggiore, Vicenza, Italy
Funding
The clinical trial was supported by Fondazione Humanitas per la Ricerca – Rozzano (MI). No other disclosures or conflicts of interest are declared.
Author information
Authors and Affiliations
Consortia
Contributions
CT, DG, and GC had full access to all data in the study and take responsibility for the integrity of the data and the accuracy of the analyses. Concept and design: CT, DG, WG, AS, EB, AT, VE, AB, EM, BF, DF, RS, RT, MS, AS, GC, and the SINODAR-ONE Collaborative Group. Acquisition, analysis, and interpretation of data: CT, DG, WG, AS, EB, AT, VE, AB, EM, CD, BD, LB, PB, GC, and the SINODAR-ONE Collaborative Group. Statistical analyses: DG, CD, BD, LB, PB. Drafting of the manuscript: DG, PB, CT, GC. Critical revision of the manuscript for important intellectual content: All authors and the SINODAR-ONE Collaborative Group. Supervision: CT, GC. Final approval of the version to be published: All authors and the SINODAR-ONE Collaborative Group.
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tinterri, C., Gentile, D., Gatzemeier, W. et al. Preservation of Axillary Lymph Nodes Compared with Complete Dissection in T1–2 Breast Cancer Patients Presenting One or Two Metastatic Sentinel Lymph Nodes: The SINODAR-ONE Multicenter Randomized Clinical Trial. Ann Surg Oncol 29, 5732–5744 (2022). https://doi.org/10.1245/s10434-022-11866-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11866-w