Abstract
Background
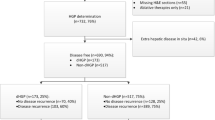
The histopathological growth pattern (HGP) of colorectal liver metastases (CLM) has been associated with prognosis. This study was designed to elucidate if the HGP is associated with local recurrence risk and impacts the adequate width of surgical margin.
Methods
All consecutive patients resected for CLM in 2018–2019 were considered. HGP was prospectively classified as follows: desmoplastic, pushing, and replacement. Surgical margin was classified as follows: R0 (margin ≥ 1 mm), R1vasc (0-mm margin, tumor detachment from intrahepatic vessels), and R1par (tumor exposure along transection plane). R0 resections were further distinguished in R0min (1-mm margin) and R0wide (> 1-mm margin).
Results
A total of 340 resection areas in 136 patients were analyzed (70 R0min, 143 R0wide, 31 R1vasc, 96 R1par). HGP was desmoplastic in 26 cases, pushing in 221, and replacement in 93. Thirty-six local recurrences occurred (11%, median follow-up 21 months): 1 after R0wide, 4 after R0min, 3 after R1vasc, and 28 after R1par resection. In R1par group, local recurrence rate was high independently of HGP (29%). In R1vasc and R0min groups, local recurrence risk was higher in the replacement group (R1vasc: 29% vs. 4% if pushing/desmoplastic; R0min: 11% vs. 4%). In R0wide group, local recurrence risk was low for all HGP ( < 1%). Independent predictors of local recurrence were replacement HGP (odds ratio = 1.654, P = 0.036), and R1par resection (odds ratio = 57.209, P < 0.001 vs. R0).
Conclusions
Replacement HGP is associated with an increased risk of local recurrence. In these patients, a wide surgical margin should be pursued, because R1vasc and R0min resections could be insufficient. R1par resection is inadequate, independently of the HGP.


Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries CA Cancer J Clin. 2020 Jul;70(4):313]. CA Cancer J Clin. 2018 68(6): 394-424. doi:https://doi.org/10.3322/caac.21492
Engstrand J, Nilsson H, Strömberg C, Jonas E, Freedman J. Colorectal cancer liver metastases - a population-based study on incidence, management and survival. BMC Cancer. 2018;18(1):78. https://doi.org/10.1186/s12885-017-3925-x.
Horn SR, Stoltzfus KC, Lehrer EJ, et al. Epidemiology of liver metastases. Cancer Epidemiol. 2020;67:101760. https://doi.org/10.1016/j.canep.2020.101760.
Viganò L, Pedicini V, Comito T, et al. Aggressive and multidisciplinary local approach to iterative recurrences of colorectal liver metastases. World J Surg. 2018;42(8):2651–9. https://doi.org/10.1007/s00268-018-4525-x.
de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250(3):440–8. https://doi.org/10.1097/SLA.0b013e3181b4539b.
Viganò L, Torzilli G, Cimino M, et al. Drop-out between the two liver resections of two-stage hepatectomy. Patient selection or loss of chance? Eur J Surg Oncol. 2016;42(9):1385–93. https://doi.org/10.1016/j.ejso.2016.03.020.
Pawlik TM, Scoggins CR, Zorzi D, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg. 2005;241(5):715–24. https://doi.org/10.1097/01.sla.0000160703.75808.7d.
Ardito F, Panettieri E, Vellone M, et al. The impact of R1 resection for colorectal liver metastases on local recurrence and overall survival in the era of modern chemotherapy: An analysis of 1,428 resection areas. Surgery. 2019;165(4):712–20. https://doi.org/10.1016/j.surg.2018.09.005.
Viganò L, Procopio F, Cimino MM, et al. Is tumor detachment from vascular structures equivalent to R0 resection in surgery for colorectal liver metastases? An Observational Cohort. Ann Surg Oncol. 2016;23(4):1352–60. https://doi.org/10.1245/s10434-015-5009-y.
Torzilli G, Donadon M, Palmisano A, et al. Ultrasound guided liver resection: does this approach limit the need for portal vein embolization? Hepatogastroenterology. 2009;56(94–95):1483–90.
Torzilli G, Procopio F, Viganò L, et al. Hepatic vein management in a parenchyma-sparing policy for resecting colorectal liver metastases at the caval confluence. Surgery. 2018;163(2):277–84. https://doi.org/10.1016/j.surg.2017.09.003.
Torzilli G, Viganò L, Gatti A, et al. Twelve-year experience of “radical but conservative” liver surgery for colorectal metastases: impact on surgical practice and oncologic efficacy. HPB (Oxford). 2017;19(9):775–84. https://doi.org/10.1016/j.hpb.2017.05.006.
Torzilli G, Serenari M, Viganò L, et al. Outcomes of enhanced one-stage ultrasound-guided hepatectomy for bilobar colorectal liver metastases compared to those of ALPPS: a multicenter case-match analysis. HPB (Oxford). 2019;21(10):1411–8.
Torzilli G, Viganò L, Cimino M, et al. Is enhanced one-stage hepatectomy a safe and feasible alternative to the two-stage hepatectomy in the setting of multiple bilobar colorectal liver metastases? A comparative analysis between two pioneering centers. Dig Surg. 2018;35(4):323–32. https://doi.org/10.1159/000486210.
Vermeulen PB, Colpaert C, Salgado R, et al. Liver metastases from colorectal adenocarcinomas grow in three patterns with different angiogenesis and desmoplasia. J Pathol. 2001;195(3):336–42. https://doi.org/10.1002/path.966.
van Dam PJ, van der Stok EP, Teuwen LA, et al. International consensus guidelines for scoring the histopathological growth patterns of liver metastasis. Br J Cancer. 2017;117(10):1427–41. https://doi.org/10.1038/bjc.2017.334.
Fernández Moro C, Bozóky B, Gerling M. Growth patterns of colorectal cancer liver metastases and their impact on prognosis: a systematic review. BMJ Open Gastroenterol. 2018;5(1):e000217. https://doi.org/10.1136/bmjgast-2018-000217.
Torzilli G, Montorsi M, Donadon M, et al. “Radical but conservative” is the main goal for ultrasonography-guided liver resection: prospective validation of this approach. J Am Coll Surg. 2005;201(4):517–28. https://doi.org/10.1016/j.jamcollsurg.2005.04.026.
Torzilli G, Montorsi M, Del Fabbro D, Palmisano A, Donadon M, Makuuchi M. Ultrasonographically guided surgical approach to liver tumours involving the hepatic veins close to the caval confluence. Br J Surg. 2006;93(10):1238–46. https://doi.org/10.1002/bjs.5321.
Vigano L, Darwish SS, Rimassa L, et al. Progression of colorectal liver metastases from the end of chemotherapy to resection: a new contraindication to surgery? Ann Surg Oncol. 2018;25(6):1676–85. https://doi.org/10.1245/s10434-018-6387-8 (Epub 2018 Feb 27 PMID: 29488188).
Torzilli G, Procopio F, Botea F, et al. One-stage ultrasonographically guided hepatectomy for multiple bilobar colorectal metastases: a feasible and effective alternative to the 2-stage approach. Surgery. 2009;146(1):60–71. https://doi.org/10.1016/j.surg.2009.02.017.
Torzilli G. Ultrasound-guided liver surgery: an Atlas. Milan: Springer-Verlag; 2014.
Viganò L, Torzilli G, Troisi R, et al. Minor hepatectomies: focusing a blurred picture: analysis of the outcome of 4471 open resections in patients without cirrhosis. Ann Surg. 2019;270(5):842–51. https://doi.org/10.1097/SLA.0000000000003493.
Viganò L, Torzilli G, Aldrighetti L, et al. Stratification of major hepatectomies according to their outcome: analysis of 2212 consecutive open resections in patients without cirrhosis. Ann Surg. 2020;272(5):827–33. https://doi.org/10.1097/SLA.0000000000004338.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. https://doi.org/10.1097/01.sla.0000133083.54934.ae.
Jass JR, Atkin WS, Cuzick J, et al. The grading of rectal cancer: historical perspectives and a multivariate analysis of 447 cases. Histopathology. 2002;41(3A):59–81.
Eefsen RL, Van den Eynden GG, Høyer-Hansen G, et al. Histopathological growth pattern, proteolysis and angiogenesis in chemo-naive patients resected for multiple colorectal liver metastases. J Oncol. 2012;2012:907971. https://doi.org/10.1155/2012/907971.
Eefsen RL, Vermeulen PB, Christensen IJ, et al. Growth pattern of colorectal liver metastasis as a marker of recurrence risk. Clin Exp Metastasis. 2015;32(4):369–81. https://doi.org/10.1007/s10585-015-9715-4.
Serrablo A, Paliogiannis P, Pulighe F, et al. Impact of novel histopathological factors on the outcomes of liver surgery for colorectal cancer metastases. Eur J Surg Oncol. 2016;42(9):1268–77. https://doi.org/10.1016/j.ejso.2016.02.013.
Nyström H, Naredi P, Berglund A, Palmqvist R, Tavelin B, Sund M. Liver-metastatic potential of colorectal cancer is related to the stromal composition of the tumor. Anticancer Res. 2012;32(12):5183–91.
Pinheiro RS, Herman P, Lupinacci RM, et al. Tumor growth pattern as predictor of colorectal liver metastasis recurrence. Am J Surg. 2014;207(4):493–8. https://doi.org/10.1016/j.amjsurg.2013.05.015.
Van den Eynden GG, Bird NC, Majeed AW, Van Laere S, Dirix LY, Vermeulen PB. The histological growth pattern of colorectal cancer liver metastases has prognostic value. Clin Exp Metastasis. 2012;29(6):541–9. https://doi.org/10.1007/s10585-012-9469-1.
Falcão D, Alexandrino H, Caetano Oliveira R, et al. Histopathologic patterns as markers of prognosis in patients undergoing hepatectomy for colorectal cancer liver metastases - Pushing growth as an independent risk factor for decreased survival. Eur J Surg Oncol. 2018;44(8):1212–9. https://doi.org/10.1016/j.ejso.2018.03.023.
Nagashima I, Oka T, Hamada C, Naruse K, Osada T, Muto T. Histopathological prognostic factors influencing long-term prognosis after surgical resection for hepatic metastases from colorectal cancer. Am J Gastroenterol. 1999;94(3):739–43. https://doi.org/10.1111/j.1572-0241.1999.00945.x.
Nielsen K, Rolff HC, Eefsen RL, Vainer B. The morphological growth patterns of colorectal liver metastases are prognostic for overall survival. Mod Pathol. 2014;27(12):1641–8. https://doi.org/10.1038/modpathol.2014.4.
Frentzas S, Simoneau E, Bridgeman VL, et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med. 2016;22(11):1294–302. https://doi.org/10.1038/nm.4197.
Liang JY, Xi SY, Shao Q, et al. Histopathological growth patterns correlate with the immunoscore in colorectal cancer liver metastasis patients after hepatectomy. Cancer Immunol Immunother. 2020;69(12):2623–34. https://doi.org/10.1007/s00262-020-02632-6.
Stremitzer S, Vermeulen P, Graver S, et al. Immune phenotype and histopathological growth pattern in patients with colorectal liver metastases. Br J Cancer. 2020;122(10):1518–24. https://doi.org/10.1038/s41416-020-0812-z.
Baldin P, Van den Eynde M, Mlecnik B, et al. Prognostic assessment of resected colorectal liver metastases integrating pathological features, RAS mutation and Immunoscore. J Pathol Clin Res. 2021;7(1):27–41. https://doi.org/10.1002/cjp2.178.
Galjart B, Nierop PMH, van der Stok EP, et al. Angiogenic desmoplastic histopathological growth pattern as a prognostic marker of good outcome in patients with colorectal liver metastases. Angiogenesis. 2019;22(2):355–68. https://doi.org/10.1007/s10456-019-09661-5.
Mentha G, Terraz S, Morel P, et al. Dangerous halo after neoadjuvant chemotherapy and two-step hepatectomy for colorectal liver metastases. Br J Surg. 2009;96(1):95–103. https://doi.org/10.1002/bjs.6436.
Nierop PMH, Galjart B, Höppener DJ, et al. Salvage treatment for recurrences after first resection of colorectal liver metastases: the impact of histopathological growth patterns. Clin Exp Metastasis. 2019;36(2):109–18. https://doi.org/10.1007/s10585-019-09960-7.
Nierop PMH, Höppener DJ, van der Stok EP, et al. Histopathological growth patterns and positive margins after resection of colorectal liver metastases. HPB (Oxford). 2020;22(6):911–9. https://doi.org/10.1016/j.hpb.2019.10.015.
Kokudo N, Miki Y, Sugai S, et al. Genetic and histological assessment of surgical margins in resected liver metastases from colorectal carcinoma: minimum surgical margins for successful resection. Arch Surg. 2002;137(7):833–40. https://doi.org/10.1001/archsurg.137.7.833.
Hamady ZZ, Lodge JP, Welsh FK, et al. One-millimeter cancer-free margin is curative for colorectal liver metastases: a propensity score case-match approach. Ann Surg. 2014;259(3):543–8. https://doi.org/10.1097/SLA.0b013e3182902b6e.
Vigano L, Di Tommaso L, Mimmo A, et al. Prospective evaluation of intrahepatic microscopic occult tumor foci in patients with numerous colorectal liver metastases. Dig Surg. 2019;36(4):340–7. https://doi.org/10.1159/000489274.
Rajaganeshan R, Prasad R, Guillou PJ, et al. The influence of invasive growth pattern and microvessel density on prognosis in colorectal cancer and colorectal liver metastases. Br J Cancer. 2007;96(7):1112–7. https://doi.org/10.1038/sj.bjc.6603677.
Fiz F, Viganò L, Gennaro N, et al. Radiomics of Liver Metastases: A Systematic Review. Cancers (Basel). 2020;12(10):2881. https://doi.org/10.3390/cancers12102881.
Costa G, Cavinato L, Masci C, et al. Virtual biopsy for diagnosis of chemotherapy-associated liver injuries and steatohepatitis: a combined radiomic and clinical model in patients with colorectal liver metastases. Cancers (Basel). 2021;13(12):3077. https://doi.org/10.3390/cancers13123077 (PMID: 34203103).
Vigano L, Sollini M, Ieva F, Fiz F, Torzilli G. Chemotherapy-associated liver injuries: unmet needs and new insights for surgical oncologists. Ann Surg Oncol. 2021;28(8):4074–9. https://doi.org/10.1245/s10434-021-10069-z (PMID: 33929618).
Cheng J, Wei J, Tong T, et al. Prediction of histopathologic growth patterns of colorectal liver metastases with a noninvasive imaging method. Ann Surg Oncol. 2019;26(13):4587–98. https://doi.org/10.1245/s10434-019-07910-x.
Han Y, Chai F, Wei J, et al. Identification of predominant histopathological growth patterns of colorectal liver metastasis by multi-habitat and multi-sequence based radiomics analysis. Front Oncol. 2020;10:1363. https://doi.org/10.3389/fonc.2020.01363.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Viganò, L., Branciforte, B., Laurenti, V. et al. The Histopathological Growth Pattern of Colorectal Liver Metastases Impacts Local Recurrence Risk and the Adequate Width of the Surgical Margin. Ann Surg Oncol 29, 5515–5524 (2022). https://doi.org/10.1245/s10434-022-11717-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11717-8