Abstract
Background
The aim of this study is to determine the optimal indications for preoperative pelvic radiotherapy (RT) in patients with metastatic rectal cancer who underwent curative-intent surgical resection and/or ablation.
Methods
Between January 2000 and October 2019, 246 patients who met our inclusion criteria were enrolled. Preoperative RT was performed in 22 patients (8.9%). Lower margin below the peritoneal reflection (p < 0.001), mesorectal fascia (MRF) invasion (p = 0.02), and lateral pelvic lymph node (LPLN) involvement (p = 0.005) were more frequent in the preoperative RT group.
Results
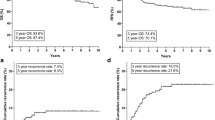
During the median follow-up period of 13.3 months (interquartile range [IQR]: 6.0–36.3 months), local recurrence (LR) was identified in 60 patients (24.4%). It was the first site of recurrence in 45 of them (18.3%). Among them, three patients were in the preoperative RT group. On multivariable analysis, lower margin below the peritoneal reflection, MRF invasion, LPLN involvement, carcinoembryonic antigen (CEA) level ≥ 10 ng/mL before treatment, and preoperative RT were significant prognostic factors for LR-free survival (LRFS). In the patient group without any risk factors, the 2-year LRFS rate was 94.9% without preoperative RT. In the patient group with one or more risk factors, the 2-year LRFS was 64.4% without and 95.2% with preoperative RT.
Conclusion
LR developed in about 25% of patients within 2 years. Preoperative RT should be considered, especially in patients with a risk factor for LR, including lower margin below the peritoneal reflection, MRF invasion, LPLN involvement, or CEA ≥ 10 ng/mL before treatment.



Similar content being viewed by others
References
Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Abate D, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol. 2019;5(12):1749–68.
Brown JJ, Asumeng CK, Greenwald D, et al. Decreased colorectal cancer incidence and mortality in a diverse urban population with increased colonoscopy screening. BMC Public Health. 2021;21(1):1280.
Cardoso R, Guo F, Heisser T, et al. Colorectal cancer incidence, mortality, and stage distribution in European countries in the colorectal cancer screening era: an international population-based study. Lancet Oncol. 2021;22(7):1002–13.
Yu WS, Bae MK, Choi JK, Hong YK, Park IK. Pulmonary metastasectomy in colorectal cancer: a population-based retrospective cohort study using the Korean National Health Insurance Database. Cancer Res Treat. 2021. https://doi.org/10.4143/crt.2020.1213 (Online Epub Jan 15).
Scherman P, Syk I, Holmberg E, Naredi P, Rizell M. Impact of patient, primary tumor and metastatic pattern including tumor location on survival in patients undergoing ablation or resection for colorectal liver metastases: a population-based national cohort study. Eur J Surg Oncol. 2021;47(2):375–83.
Kang ST, Moran R, Hussain L, Guend H, Dunki-Jacobs EM, Lee DY. Metastasectomy for stage IVA colon cancer: does the type of treating institution make a difference? Am Surg. 2021. https://doi.org/10.1177/00031348211023389 (Online Epub June 2).
Van Cutsem E, Cervantes A, Adam R, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. 2016;27(8):1386–422.
Yoshino T, Arnold D, Taniguchi H, et al. Pan-Asian adapted ESMO consensus guidelines for the management of patients with metastatic colorectal cancer: a JSMO-ESMO initiative endorsed by CSCO, KACO, MOS, SSO and TOS. Ann Oncol. 2018;29(1):44–70.
Butte JM, Gonen M, Ding P, et al. Patterns of failure in patients with early onset (synchronous) resectable liver metastases from rectal cancer. Cancer. 2012;118(21):5414–23.
Folkesson J, Birgisson H, Pahlman L, Cedermark B, Glimelius B, Gunnarsson U. Swedish Rectal Cancer Trial: long lasting benefits from radiotherapy on survival and local recurrence rate. J Clin Oncol. 2005;23(24):5644–50.
van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol. 2011;12(6):575–82.
Wolmark N, Wieand HS, Hyams DM, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst. 2000;92(5):388–96.
Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med. 2008;359(17):1757–65.
Heinemann V, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15(10):1065–75.
Douillard JY, Oliner KS, Siena S, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. 2013;369(11):1023–34.
Bokemeyer C, Bondarenko I, Makhson A, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27(5):663–71.
Moslim MA, Bastawrous AL, Jeyarajah DR. Neoadjuvant pelvic radiotherapy in the management of rectal cancer with synchronous liver metastases: is it worth it? J Gastrointest Surg. 2021. https://doi.org/10.1007/s11605-021-05042-w (Online Epub June 7).
Benson AB, Venook AP, Al-Hawary MM, et al. NCCN Guidelines insights: rectal cancer, version 6.2020. J Natl Compr Canc Netw. 2020;18(7):806–15.
Park JW, Kang SB, Hao J, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): 10-year follow-up of an open-label, non-inferiority, randomised controlled trial. Lancet Gastroenterol Hepatol. 2021;6(7):569–77.
Wang J, Long Y, Liu K, Pei Q, Zhu H. Comparing neoadjuvant long-course chemoradiotherapy with short-course radiotherapy in rectal cancer. BMC Gastroenterol. 2021;21(1):277.
Schrag D, Weiser MR, Goodman KA, et al. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32(6):513–8.
Holliday EB, Hunt A, You YN, et al. Short course radiation as a component of definitive multidisciplinary treatment for select patients with metastatic rectal adenocarcinoma. J Gastrointest Oncol. 2017;8(6):990–7.
Kim KH, Shin SJ, Cho MS, et al. A phase II study of preoperative mFOLFOX6 with short-course radiotherapy in patients with locally advanced rectal cancer and liver-only metastasis. Radiother Oncol. 2016;118(2):369–74.
Yoon HI, Koom WS, Kim TH, et al. Upfront systemic chemotherapy and short-course radiotherapy with delayed surgery for locally advanced rectal cancer with distant metastases: outcomes, compliance, and favorable prognostic factors. PLoS ONE. 2016;11(8):e0161475.
Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):29–42.
Acknowledgment
We would like to thank Editage (www.editage.co.kr) for English language editing.
Funding
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education (NRF-2020R1F1A1073205).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
DISCLOSURE
The authors declare that they have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, J.I., Yoo, G.S., Park, H.C. et al. Determining Which Patients Require Preoperative Pelvic Radiotherapy Before Curative-Intent Surgery and/or Ablation for Metastatic Rectal Cancer. Ann Surg Oncol 29, 4197–4207 (2022). https://doi.org/10.1245/s10434-022-11592-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11592-3