Abstract
Background
Patients with T4 colon adenocarcinomas have an increased risk of peritoneal metastases (PM) but the histopathologic risk factors for its development are not well-described.
Objective
The purpose of this study was to determine factors associated with PM, time to recurrence, and survival after recurrence among patients with T4 colon cancer.
Patients and Methods
Patients with pathologic T4 colon cancer who underwent curative resection from 2005 to 2017 were identified from a prospectively maintained institutional database and classified by recurrence pattern: (a) none – 68.8%; (b) peritoneal only – 7.9%; (c) peritoneal and extraperitoneal – 9.9%; and (d) extraperitoneal only – 13.2%. Associations between PM development and patient, primary tumor, and treatment factors were assessed.
Results
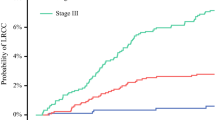
Overall, 151 patients were analyzed, with a median follow-up of 66.2 months; 27 patients (18%) developed PM (Groups B and C) and 20 (13%) patients recurred at non-peritoneal sites only (Group D). Median time to developing metastases was shorter for Groups B and C compared with Group D (B and C: 13.7 months; D: 46.7 months; p = 0.022). Tumor deposits (TDs) and nodal stage were associated with PM (p < 0.05), and TDs (p = 0.048) and LVI (p = 0.015) were associated with additional extraperitoneal recurrence. Eleven (41%) patients with PM underwent salvage surgery, and median survival after recurrence was associated with the ability to undergo cytoreduction (risk ratio 0.20, confidence interval 0.06–0.70).
Conclusion
PM risk after resection of T4 colon cancer is independently associated with factors related to lymphatic spread, such as N stage and TDs. Well-selected patients can undergo cytoreduction with long-term survival. These findings support frequent postoperative surveillance and aggressive early intervention, including cytoreduction.



Similar content being viewed by others
References
Virostko J, Capasso A, Yankeelov TE, Goodgame B. Recent trends in the age at diagnosis of colorectal cancer in the US National Cancer Data Base, 2004–2015. Cancer. 2019;125(21):3828–35.
Healy MA, Peacock O, Hu CY, et al. High rate of positive circumferential resection margin in colon cancer: A national appraisal and call for action. Ann Surg. Epub 22 Dec 2020. https://doi.org/10.1097/SLA.0000000000004682
Shepherd NA, Baxter KJ, Love SB. The prognostic importance of peritoneal involvement in colonic cancer: a prospective evaluation. Gastroenterology. 1997;112(4):1096–102.
Elferink MA, de Jong KP, Klaase JM, Siemerink EJ, de Wilt JH. Metachronous metastases from colorectal cancer: a population-based study in North-East Netherlands. Int J Colorectal Dis. 2015;30(2):205–12.
Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99(5):699–705.
Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JW, de Hingh IH. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer. 2011;128(11):2717–25.
Segelman J, Akre O, Gustafsson UO, Bottai M, Martling A. Individualized prediction of risk of metachronous peritoneal carcinomatosis from colorectal cancer. Colorectal Dis. 2014;16(5):359–67.
Klaver CEL, Wisselink DD, Punt CJA, et al. Adjuvant hyperthermic intraperitoneal chemotherapy in patients with locally advanced colon cancer (COLOPEC): a multicentre, open-label, randomised trial. Lancet Gastroenterol Hepatol. 2019;4(10):761–70.
van Santvoort HC, Braam HJ, Spekreijse KR, et al. Peritoneal carcinomatosis in t4 colorectal cancer: occurrence and risk factors. Ann Surg Oncol. 2014;21(5):1686–91.
Franko J, Shi Q, Meyers JP, et al. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: an analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016;17(12):1709–19.
Kitano S, Inomata M, Mizusawa J, et al. Survival outcomes following laparoscopic versus open D3 dissection for stage II or III colon cancer (JCOG0404): a phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol. 2017;2(4):261–8.
Yamanashi T, Nakamura T, Sato T, et al. Laparoscopic surgery for locally advanced T4 colon cancer: the long-term outcomes and prognostic factors. Surg Today. 2018;48(5):534–44.
Lu J, Dong B, Yang Z, et al. Clinical Efficacy of Laparoscopic Surgery for T4 Colon Cancer Compared with Open Surgery: A Single Center’s Experience. J Laparoendosc Adv Surg Tech A. 2019;29(3):333–9.
Aoki T, Matsuda T, Hasegawa H, et al. Outcomes of laparoscopic surgery for pathological T4 colon cancer. Int J Colorectal Dis. 2019;34(7):1259–65.
Khan MA, Hakeem AR, Scott N, Saunders RN. Significance of R1 resection margin in colon cancer resections in the modern era. Colorectal Dis. 2015;17(11):943–53.
Leon P, Iovino MG, Giudici F, et al. Oncologic outcomes following laparoscopic colon cancer resection for T4 lesions: a case-control analysis of 7-years’ experience. Surg Endosc. 2018;32(3):1133–40.
Macari D, Kawak S, Raofi V, Wasvary H, Jaiyesimi I. Recurrence pattern and outcomes in T4 colon cancer: a single institution analysis. J Surg Oncol. Epub 14 Nov 2019. https://doi.org/10.1002/jso.25766
van Gestel YR, Thomassen I, Lemmens VE, et al. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur J Surg Oncol. 2014;40(8):963–9.
Riihimaki M, Hemminki A, Sundquist J, Hemminki K. Patterns of metastasis in colon and rectal cancer. Sci Rep. 2016;6:29765.
Quenet F, Elias D, Roca L, et al. Cytoreductive surgery plus hyperthermic intraperitoneal chemotherapy versus cytoreductive surgery alone for colorectal peritoneal metastases (PRODIGE 7): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2021;22(2):256–66.
Thomassen I, van Gestel YR, Lemmens VE, de Hingh IH. Incidence, prognosis, and treatment options for patients with synchronous peritoneal carcinomatosis and liver metastases from colorectal origin. Dis Colon Rectum. 2013;56(12):1373–80.
Liebig C, Ayala G, Wilks J, et al. Perineural invasion is an independent predictor of outcome in colorectal cancer. J Clin Oncol. 2009;27(31):5131–7.
Tsai HL, Chu KS, Huang YH, et al. Predictive factors of early relapse in UICC stage I-III colorectal cancer patients after curative resection. J Surg Oncol. 2009;100(8):736–43.
Lim SB, Yu CS, Jang SJ, Kim TW, Kim JH, Kim JC. Prognostic significance of lymphovascular invasion in sporadic colorectal cancer. Dis Colon Rectum. 2010;53(4):377–84.
Chuang SC, Su YC, Lu CY, et al. Risk factors for the development of metachronous liver metastasis in colorectal cancer patients after curative resection. World J Surg. 2011;35(2):424–9.
Lehnert T, Methner M, Pollok A, Schaible A, Hinz U, Herfarth C. Multivisceral resection for locally advanced primary colon and rectal cancer: an analysis of prognostic factors in 201 patients. Ann Surg. 2002;235(2):217–25.
Croner RS, Merkel S, Papadopoulos T, Schellerer V, Hohenberger W, Goehl J. Multivisceral resection for colon carcinoma. Dis Colon Rectum. 2009;52(8):1381–6.
Snaebjornsson P, Coupe VM, Jonasson L, Meijer GA, van Grieken NC, Jonasson JG. pT4 stage II and III colon cancers carry the worst prognosis in a nationwide survival analysis. Shepherd's local peritoneal involvement revisited. Int J Cancer. Jul 2014;135(2):467-478.
Nagata H, Kawai K, Hata K, Tanaka T, Nozawa H, Ishihara S. Laparoscopic surgery for T4 colon cancer: a risk factor for peritoneal recurrences? Surgery. 2020;168(1):119–24.
Hasegawa H, Okabayashi K, Watanabe M, et al. What is the effect of laparoscopic colectomy on pattern of colon cancer recurrence? A propensity score and competing risk analysis compared with open colectomy. Ann Surg Oncol. 2014;21(8):2627–35.
Feinberg AE, Chesney TR, Acuna SA, Sammour T, Quereshy FA. Oncologic Outcomes Following Laparoscopic versus Open Resection of pT4 Colon Cancer: A Systematic Review and Meta-analysis. Dis Colon Rectum. 2017;60(1):116–25.
Franiel T, Diederichs G, Engelken F, Elgeti T, Rost J, Rogalla P. Multi-detector CT in peritoneal carcinomatosis: diagnostic role of thin slices and multiplanar reconstructions. Abdom Imaging. 2009;34(1):49–54.
Marin D, Catalano C, Baski M, et al. 64-Section multi-detector row CT in the preoperative diagnosis of peritoneal carcinomatosis: correlation with histopathological findings. Abdom Imag. 2010;35(6):694–700.
Dohan A, Hoeffel C, Soyer P, et al. Evaluation of the peritoneal carcinomatosis index with CT and MRI. Br J Surg. 2017;104(9):1244–9.
Wille-Jorgensen P, Syk I, Smedh K, et al. Effect of More vs Less Frequent Follow-up Testing on Overall and Colorectal Cancer-Specific Mortality in Patients With Stage II or III Colorectal Cancer: The COLOFOL Randomized Clinical Trial. JAMA. 2018;319(20):2095–103.
Snyder RA, Hu CY, Cuddy A, et al. Association Between Intensity of Posttreatment Surveillance Testing and Detection of Recurrence in Patients With Colorectal Cancer. JAMA. 2018;319(20):2104–15.
Schmidt S, Meuli RA, Achtari C, Prior JO. Peritoneal carcinomatosis in primary ovarian cancer staging: comparison between MDCT, MRI, and 18F-FDG PET/CT. Clin Nucl Med. 2015;40(5):371–7.
Anandappa G, Starling N, Peckitt C, et al. TRACC: Tracking mutations in cell-free DNA to predict relapse in early colorectal cancer—a randomized study of circulating tumour DNA (ctDNA) guided adjuvant chemotherapy versus standard of care chemotherapy after curative surgery in patients with high risk stage II or stage III colorectal cancer (CRC). Journal of Clinical Oncology. 2020;38(15 Suppl):TPS4120.
Morris VK, Yothers G, Kopetz S, et al. Phase II/III study of circulating tumor DNA as a predictive biomarker in adjuvant chemotherapy in patients with stage II colon cancer:NRG-GI005 (COBRA). Journal of Clinical Oncology. 2021;39(3 Suppl):TPS148.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Abhineet Uppal, Beth Helmink, Travis E. Grotz, Tsuyoshi Konishi, Keith F. Fournier, Sa Nguyen, Melissa W. Taggart, John Paul Shen, Brian K. Bednarski, Yi-Qian N. You, and George J. Chang report no conflicts of interest related to the contents of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Uppal, A., Helmink, B., Grotz, T.E. et al. What is the Risk for Peritoneal Metastases and Survival Afterwards in T4 Colon Cancers?. Ann Surg Oncol 29, 4224–4233 (2022). https://doi.org/10.1245/s10434-022-11472-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-022-11472-w