Abstract
Purpose
Talimogene Laherparepvec (T-VEC) is a modified herpes simplex virus type-1 used as intralesional immunotherapy in stage IIIB-IVM1a melanoma patients. Recently, Stahlie et al. published a predictive model for complete response (CR) to T-VEC. This study was designed to validate this model externally in an independent, American patient cohort.
Methods
In total, 71 stage IIIB-IVM1a melanoma patients treated with T-VEC at Moffitt Cancer Center were included. A second nomogram was built incorporating the same predictive factors: tumor size (diameter of largest metastasis), type of metastases (cutaneous, subcutaneous and nodal), and number of metastases (cutoff: < 20 and > 20). Predictive accuracy was assessed through calculation of overall performance, discriminative ability, and calibration.
Results
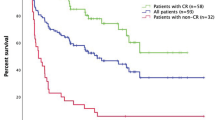
The two cohorts were similar in many clinicopathologic factors and only differing in tumor mutational status and use of systemic therapy prior to T-VEC. In the validation cohort, 37 (52%) patients showed CR, 22 (31%) partial response (PR), 2 (5.6%) stable disease (SD), and 10 (15%) progressive disease (PD). Of those who demonstrated a CR, 16 (43%) recurred. Overall performance was good (0.164), and discriminative power resulted in fair discriminative ability (0.827). The calibration curve showed slight underestimation for predicted probabilities > 0.15 and slight overestimation <0.15.
Conclusions
The original model as well as the validation model show comparable and good predictive accuracy. The validation model reinforces the conclusion that for the best response to T-VEC, it should be used early on in the course of the disease, when the tumor burden is cutaneous with smaller diameter and fewer of metastases.


Similar content being viewed by others
References
Kaufman HL, Bines SD. OPTIM trial: a Phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncol. 2010;6(6):941–9.
Rehman H, Silk AW, Kane MP, et al. Into the clinic: Talimogene laherparepvec (T-VEC), a first-in-class intratumoral oncolytic viral therapy. J Immunother Cancer. 2016;4:53.
Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene Laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–8.
Franke V, Berger DMS, Klop WMC, et al. High response rates for T-VEC in early metastatic melanoma (stage IIIB/C-IVM1a). Int J Cancer. 2019;145(4):974–8.
Stahlie EHA, Franke V, Zuur CL, et al. T-VEC for stage IIIB-IVM1a melanoma achieves high rates of complete and durable responses and is associated with tumor load: a clinical prediction model. Cancer Immunol Immunother. 2021;70(8):2291–300.
Perez MC, Miura JT, Naqvi SMH, et al. Talimogene Laherparepvec (TVEC) for the treatment of advanced melanoma: a single-institution experience. Ann Surg Oncol. 2018;25(13):3960–5.
Mohr P, Haferkamp S, Pinter A, et al. Real-world use of talimogene laherparepvec in German patients with stage IIIB to IVM1a melanoma: a retrospective chart review and physician survey. Adv Ther. 2019;36(1):101–17.
Amgen. Imlygic (talimogene laherperepvec) suspension for intralesional injection: US prescribing information. 2015.
Harrington KJ, Michielin O, Malvehy J, et al. A practical guide to the handling and administration of talimogene laherparepvec in Europe. Oncol Targets Ther. 2017;10:3867–80.
Tetzlaff MT, Messina JL, Stein JE, et al. Pathological assessment of resection specimens after neoadjuvant therapy for metastatic melanoma. Ann Oncol. 2018;29(8):1861–8.
Geneva W, Organization WH, Organization WH. WHO Handbook for reporting results of cancer treatment. WHO Offset Publication. 1979;(48).
Balachandran VP, Gonen M, Smith JJ, et al. Nomograms in oncology: more than meets the eye. Lancet Oncol. 2015;16(4):e173–80.
Zhou AY, Wang DY, McKee S, et al. Correlates of response and outcomes with talimogene laherperpvec. J Surg Oncol. 2019;120(3):558–64.
Masoud SJ, Hu JB, Beasley GM, et al. Efficacy of Talimogene Laherparepvec (T-VEC) therapy in patients with in-transit melanoma metastasis decreases with increasing lesion size. Ann Surg Oncol. 2019;26(13):4633–41.
Chang CJ, Tai KF, Roffler S, et al. The immunization site of cytokine-secreting tumor cell vaccines influences the trafficking of tumor-specific T lymphocytes and antitumor efficacy against regional tumors. J Immunol. 2004;173(10):6025–32.
Li H, van der Leun AM, Yofe I, et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell. 2020;181(3):747.
Dong XD, Tyler D, Johnson JL, et al. Analysis of prognosis and disease progression after local recurrence of melanoma. Cancer. 2000;88(5):1063–71.
Ressler JM, Karasek M, Koch L, et al. Real-life use of talimogene laherparepvec (T-VEC) in melanoma patients in centers in Austria, Switzerland and Germany. J Immunother Cancer. 2021;9(2):e001701.
Puzanov I, Milhem MM, Minor D, et al. Talimogene Laherparepvec in Combination With Ipilimumab in Previously Untreated, Unresectable Stage IIIB-IV Melanoma. J Clin Oncol. 2016;34(22):2619–26.
Long G, Dummer R, Andtbacka R, et al. Follow-up analysis of MASTERKEY-265 phase 1b (ph1b) study of talimogene laherparepvec (T-VEC) in combination (combo) with pembrolizumab (pembro) in patients (pts) with unresectable stage IIIB–IVM1c melanoma (MEL). Society for Melanoma Research Fifteenth International Congress, 2018.
Carr MJSJ, DePalo D, Rothermel LD, Song Y, Straker RJ, Baecher K, Louie RJ, Stahlie EHA, Wright GP, Naqvi SMH, Kim Y, Sarnaik AA, Karakousis GC, Lowe MC, Delman KA, van Akkooi ACJ, Ollila DW, Collichio F, Zager JS. Talimogene Laherparepvec (TVEC) for the treatment of advanced locoregional melanoma after failure of immunotherapy: a multi-institutional experience. Ann Surg Oncol. 2021. https://doi.org/10.1245/s10434-021-10910-5.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Jonathan S. Zager reports grants from Amgen, Delcath Systems, Philogen, and Provectus; grants and personal fees from Castle Biosciences and Novartis, and personal fees from Pfizer, Merck, and Sun Pharma. Alexander van Akkooi reports advisory board and consultancy honoraria (all paid to institute and unrelated to current work) from Amgen, Bristol- Myers Squibb, Novartis; 4SC, Merck- Pfizer, MSD- Merck, Sanofi and Sirius medical. The remaining authors made no disclosures.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Stahlie, E.H.A., Carr, M.J., Zager, J.S. et al. External Validation of a Dutch Predictive Nomogram for Complete Response to T-VEC in an Independent American Patient Cohort. Ann Surg Oncol 29, 1637–1644 (2022). https://doi.org/10.1245/s10434-021-11111-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-11111-w