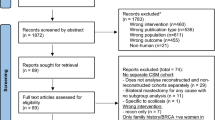
Abstract
Background
Reconstructive techniques for restoring sensation to the breast after mastectomy continue to evolve. The BREAST-Q is a patient-reported outcome measure that can be used to evaluate outcomes of breast cancer treatments; however, it previously lacked scales to measure breast sensation. This paper outlines the development and validation of the BREAST-Q Sensation Module.
Methods
Phase 1 (January 2017 through December 2018) involved qualitative and cognitive interviews with women who had undergone breast reconstruction, as well as expert input, to develop and refine the scales. In phase 2 (March through June 2019), Love Research Army (LRA) members completed the scales, and Rasch Measurement Theory (RMT) analysis was performed to examine the reliability and validity of the scales.
Results
In this study, 36 qualitative and 7 cognitive interviews were conducted, and input from 18 experts was obtained. Three scales were developed to measure breast Symptoms (e.g., throbbing, burning, tingling), Sensation (e.g., feeling with light touch, through clothing, sexually), and Quality of Life impact of sensation loss. In phase 2, 1204 LRA members completed the scales. Data for each scale fit the RMT model. Reliability was high, with Person Separation Index, Cronbach alpha, and intraclass correlation coefficient values of 0.81 or higher (with and without extremes) for all three scales. Mean scores were higher (better) on the Symptoms and Quality of Life impact scales for the participants with unilateral (vs. bilateral) and autologous (vs. alloplastic) reconstruction, and for the participants who were farther out from their reconstruction.
Conclusion
The BREAST-Q Sensation Module can be used alone or in conjunction with other BREAST-Q scales to inform clinical care and to evaluate outcomes of new surgical approaches to restoration of breast sensation.





Similar content being viewed by others
References
Engel J, Kerr J, Schlesinger-Raab A. Quality of life following breast-conserving therapy or mastectomy: results of a 5-year prospective study. Breast J. 2004;10:223–31.
American Cancer Society. Breast Cancer Facts and Figures 2017–2018. https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/breast-cancer-facts-and-figures/breast-cancer-facts-and-figures-2017-2018.pdf. Accessed 14 Oct 2020.
Koçan S, Gürsoy A. Body image of women with breast cancer after mastectomy: a qualitative research. J Breast Health. 2016;12:145–50.
Faulkner HR, Colwell AS, Liao EC, et al. Thermal injury to reconstructed breasts from commonly used warming devices: a risk for reconstructive failure. PRS GO. 2016;4:e1033.
Cornelissen AJM, Beugels J, van Kuijk SMJ, et al. Sensation of the autologous reconstructed breast improves quality of life: a pilot study. Breast Cancer Res Treat. 2018;167:687–95.
Peled AW, Peled ZM. Nerve preservation and allografting for sensory innervation following immediate implant breast reconstruction. Plast Reconstr Surg Glob Open. 2019;7:e2332.
Djohan R, Knackstedt R, Scomacao I, et al. A novel approach to sensory re-innervation to the nipple areolar complex after mastectomy with implant-based reconstruction: anatomic and technical considerations. J Plast Reconstr Aesthet Surg. 2020;73:983–1007.
Shridharani SM, Magarakis M, Stapleton SM, et al. Breast sensation after breast reconstruction: a systematic review. J Reconstr Microsurg. 2010;26:303–10.
Blondeel PN, Demuynck M, Mete D, et al. Sensory nerve repair in perforator flaps for autologous breast reconstruction: sensational or senseless? Br J Plast Surg. 1999;52:37–44.
Lapatto O, Asko-Selijavaara S, Tukiainen E, et al. Return of sensibility and final outcome of breast reconstructions using free transverse rectus abdominis musculocutaneous flaps. Scand J Plast Reconstr Surg Hand Surg. 1995;29:33–8.
Lehmann C, Gumenere R, Montandon D. Sensibility and cutaneous reinnervation after breast reconstruction with musculocutaneous flaps. Ann Plast Surg. 1991;26:325–7.
Place MJ, Song T, Hardesty RA, et al. Sensory re-innervation of autologous tissue TRAM flaps after breast reconstruction. Ann Plast Surg. 1997;38:19–22.
Shaw WW, Orringer JS, Ko CY, et al. The spontaneous return of sensibility in breasts reconstructed with autologous tissue. Plast Reconstr Surg. 1997;99:394–9.
Edsander-Nord A, Wickman M, Hansson P. Somatosensory status after pedicled or free TRAM flap surgery: a retrospective study. Plast Reconstr Surg. 1999;104:1642–8.
Yap LH, Whiten SC, Forster A, et al. Sensory recovery in the sensate free transverse rectus abdominis myocutaneous flap. Plast Reconstr Surg. 2005;115:1280–8.
Temple CL, Tse R, Bettger-Hahn M, et al. Sensibility following innervated free TRAM flap for breast reconstruction. Plast Reconstr Surg. 2006;117:2119–27.
Isenberg JS, Spinelli H. Further experience with innervated autologous flaps in postoncologic breast reconstruction. Ann Plast Surg. 2004;52:448–51.
Weissler JM, Koltz PF, Carney MJ, et al. Sifting through the evidence: a comprehensive review and analysis of neurotization in breast reconstruction. Plast Reconstr Surg. 2017;141:550–65.
Beugels J, Cornelissen AJM, Spiegel AJ, et al. Sensory recovery of the breast after innervated and non-innervated autologous breast reconstructions: a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:1229–41.
Zhou A, Ducic I, Momeni A. Sensory restoration of breast reconstruction: the search for the ideal approach continues. J Surg Oncol. 2018;118:780–92.
Pusic AL, Klassen AF, Scott AM, et al. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124:345–53.
Weissler JM, Koltz PF, Carney MJ, et al. Sifting through the evidence: a comprehensive review and analysis of neurotization in breast reconstruction. Plast Reconstr Surg. 2018;141:550–65.
Aaronson N, Alonso J, Burnam A, et al. Assessing health status and quality-of-life instruments: attributes and review criteria. Qual Life Res. 2002;11:193–205.
U.S. Department of Health and Human Services. Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. https://www.fda.gov/media/77832/download. Accessed 14 Oct 2020.
Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity: establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO good research practices task force report: part 1. Eliciting concepts for a new PRO instrument. Value Health. 2011;14:967–77.
Patrick DL, Burke LB, Gwaltney CJ, et al. Content validity: establishing and reporting the evidence in newly developed patient-reported outcomes (PRO) instruments for medical product evaluation: ISPOR PRO Good Research Practices Task Force report: part 2. Assessing respondent understanding. Value Health. 2011;14:978–88.
Terwee CB, Prinsen CAC, Chiarotto A, et al. COSMIN methodology for assessing the content validity of PROMs user manual. Amsterdam: VU University Medical Center; 2018.
Terwee CB, Prinsen CAC, Chiarotto A, et al. COSMIN methodology for evaluating the content validity of patient-reported outcome measures: a Delphi study. Qual Life Res. 2018;27:1159–70.
Thorne S, Kirkham SR, MacDonald-Emes J. Interpretive description: a noncategorical qualitative alternative for developing nursing knowledge. Res Nurs Health. 1997;20:169–77.
Thorne SE. Interpretive description: developing qualitative inquiry. vol 2, Walnut Creek: Left Coast Press; 2008.
Willis GB. Cognitive interviewing in practice: think-aloud, verbal probing, and other techniques—cognitive interviewing. Thousand Oaks: Sage Publications; 2005. p. 42–65.
Collins D. Pretesting survey instruments: an overview of cognitive methods. Qual Life Res. 2003;12:229–38.
Van Someren M, Barnard Y, Sandberg J. The think-aloud method: a practical approach to modelling cognitive. London: Academic Press; 1994.
Willis GB, Artino AR Jr. What do our respondents think we’re asking? Using cognitive interviewing to improve medical education surveys. J Grad Med Educ. 2013;5:353–6.
García AA. Cognitive interviews to test and refine questionnaires. Public Health Nurs. 2011;28:444–50.
Willis GB. Cognitive interviewing: a tool for improving questionnaire design. New York: Sage Publications; 2005.
Willis GB. Analysis of the cognitive interview in questionnaire design: understanding qualitative research. Toronto: Oxford University Press; 2015.
Miller K. Cognitive interviewing. In: J Madans, K Miller, A Maitland, G Willis, editors. Question evaluation methods. Hoboken: Wiley; 2011. p. 51–75.
Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81.
Kincaid JP, Fishburne RP, Rogers RL, Chissom BS. Derivation of new readability formulas (Automated Readability Index, Fog count, and Flesch reading ease formula) for Navy enlisted personnel. Research Branch Report 8–75. Chief of Naval Technical Training: Naval Air Station Memphis (1975).
Love S. Love Research Army. https://www.armyofwomen.org/. Accessed 14 Oct 2020.
Rasch G. Probabilistic models for some intelligence and attainment tests. Studies in mathematical psychology. vol 1, Copenhagen: Danmarks Paedagogiske Institut; 1960.
Hobart J, Cano S. Improving the evaluation of therapeutic intervention in multiple sclerosis: the role of new psychometric methods. Health Technol Assess. 2009;13:1–200.
Andrich D. Rasch models for measurement. Sage university papers series quantitative applications in the social sciences. vol 07–068, Newbury Park: Sage; 1988.
Wright BD, Masters GN. Rating scale analysis. Chicago: MESA Press; 1982.
Andrich D, Luo G, Sheridan BE. Interpreting RUMM2030. Perth: RUMM Laboratory; 2016.
Cronbach LJ. Coefficient alpha and the internal structure of tests. Psychometrika. 1951;16:297–334.
Nunnally JC. Psychometric theory. 3rd edn. New York: McGraw-Hill; 1994.
Andrich D. An elaboration of Guttman scaling with Rasch models for measurement. Soc Methodol. 1985;15:33–80.
Marais I. Local dependence. In: KB Christensen, S Kreiner, M Mesbah, editors. Rasch models in health. London: Wiley; 2013. p. 111–30.
Terwee CB, Bot SDM, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol. 2007;60:34–42.
Prinsen CA, Mokkink LB, Bouter LM, et al. COSMIN guideline for systematic reviews of patient-reported outcome measures. Qual Life Res. 2018;27:1147–57.
Kim H-Y. Statistical notes for clinical researchers: assessing normal distribution (2) using skewness and kurtosis. Restor Dent Endod. 2013;38:52–4.
Humble SR, Dalton AJ, Li L. A systematic review of therapeutic interventions to reduce acute and chronic post-surgical pain after amputation, thoracotomy or mastectomy. Eur J Pain. 2015;19:451–65.
Jager J, Putnick DL, Bornstein MH. More than just convenient: the scientific merits of homogeneous convenience samples. Monogr Soc Res Child Dev. 2017;82:13–30.
Bornstein MH, Jager J, Putnick DL. Sampling in developmental science: situations, shortcomings, solutions, and standards. Dev Rev. 2013;33:357–70.
Acknowledgment
Phases 1 and 2 of this study were supported by the Canadian Breast Cancer Foundation Project Grant (now integrated into Canadian Cancer Society) (Grant No. 319371). Phase 2 of this study was supported by the Plastic Surgery Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The BREAST-Q Sensation Module is owned by Memorial Sloan-Kettering Cancer Center, McMaster University, and Mass General Brigham, and Drs Pusic and Klassen are co-developers. The remaining authors have no conflicts of interest. Drs Anne Klassen, Toni Zhong, and Manraj Kaur are grateful for funding from the Canadian Institutes of Health Research (CIHR). Dr. Kaur holds a CIHR Training Award. The funders were not involved in the design of the study, data collection or analysis, decision to publish, or preparation of the manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tsangaris, E., Klassen, A.F., Kaur, M.N. et al. Development and Psychometric Validation of the BREAST-Q Sensation Module for Women Undergoing Post-Mastectomy Breast Reconstruction. Ann Surg Oncol 28, 7842–7853 (2021). https://doi.org/10.1245/s10434-021-10094-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-021-10094-y