Abstract
Background
Most minorities receive cancer care at minority-serving hospitals (MSHs) that have been associated with disparate treatment between Black and White patients.
Objective
Our aim was to examine the uptake of clinical trials that have changed axillary management in breast cancer patients at MSH and non-MSH cancer centers.
Methods
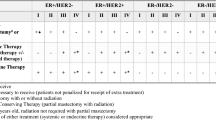
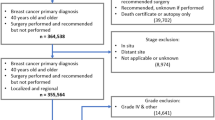
The National Cancer Database was used to identify patients eligible for the American College of Surgeons Oncology Group Z0011 and Z1071 trials, and mastectomy patients fulfilling the European AMAROS trial. Uptake of trial results (omission of axillary lymph node dissection) was analyzed between patients treated at MSHs and non-MSHs and adjusted for patient, tumor, and facility factors. MSHs were defined as the top decile of hospitals according to the proportion of Black and Hispanic patients treated.
Results
Of 7167 patients eligible for Z0011, 4546 for Z0171, and 9433 for AMAROS from 2015 to 2016, clinical trial uptake was seen in 1195 (74.6%) MSH and 4056 (72.9%) non-MSH patients (p = 0.173) for Z0011, 588 (41.9%) MSH and 1366 (43.5%) non-MSH patients for Z1071 (p = 0.302), and 272 (11.7%) MSH and 996 (14.0%) non-MSH patients (p = 0.005) for AMAROS. On adjusted analyses, MSH status was not significant for uptake of any of the three trials. Black race, socioeconomic status, and insurance were not associated with clinical trial uptake.
Conclusion
The uptake of three landmark clinical trials of axillary management in breast cancer was not different at MSH and non-MSH centers despite adjustment for social determinants of health. At the Commission on Cancer-accredited centers in this analysis, MSH status did not affect the uptake of evidence-based care.


Similar content being viewed by others
References
National Healthcare Quality and Disparities Report. Agency for Healthcare Research and Quality, Rockville, MD. https://www.ahrq.gov/research/findings/nhqrdr/nhqdr18/index.html. Accessed Apr 2020.
Singh GK, Jemal A. Socioeconomic and racial/ethnic disparities in cancer mortality, incidence, and survival in the United States, 1950–2014: over six decades of changing patterns and widening inequalities. J Environ Public Health. 2017;2017:2819372.
Singh GK, Williams SD, Siahpush M, Mulhollen A. Socioeconomic, rural-urban, and racial inequalities in US cancer mortality: part I-all cancers and lung cancer and part II-colorectal, prostate, breast, and cervical cancers. J Cancer Epidemiol. 2011;2011:107497.
Ellis L, Canchola AJ, Spiegel D, Ladabaum U, Haile R, Gomez SL. Racial and ethnic disparities in cancer survival: the contribution of tumor, sociodemographic, institutional, and neighborhood characteristics. J Clin Oncol. 2018;36(1):25–33.
DeSantis CE, Ma J, Gaudet MM, et al. Breast cancer statistics, 2019. CA Cancer J Clin. 2019;69(6):438–451.
DeSantis CE, Ma J, Goding Sauer A, Newman LA, Jemal A. Breast cancer statistics, 2017, racial disparity in mortality by state. CA Cancer J Clin. 2017;67(6):439–448.
Jemal A, Robbins AS, Lin CC, et al. Factors that contributed to black-white disparities in survival among nonelderly women with breast cancer between 2004 and 2013. J Clin Oncol. 2018;36(1):14–24.
Ko NY, Hong S, Winn RA, Calip GS. Association of insurance status and racial disparities with the detection of early-stage breast cancer. JAMA Oncol. 2020;6(3):385–392.
Yedjou CG, Tchounwou PB, Payton M, et al. Assessing the racial and ethnic disparities in breast cancer mortality in the United States. Int J Environ Res Public Health. 2017;14(5):486.
Freedman RA, Virgo KS, He Y, et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117(1):180–189.
Keating NL, Kouri E, He Y, Weeks JC, Winer EP. Racial differences in definitive breast cancer therapy in older women: are they explained by the hospitals where patients undergo surgery? Med Care. 2009;47(7):765–773.
Keating NL, Kouri EM, He Y, Freedman RA, Volya R, Zaslavsky AM. Location isn’t everything: proximity, hospital characteristics, choice of hospital, and disparities for breast cancer surgery patients. Health Serv Res. 2016;51(4):1561–1583.
Krimphove MJ, Fletcher SA, Cole AP, et al. Quality of care in the treatment of localized intermediate and high risk prostate cancer at minority serving hospitals. J Urol. 2019;201(4):735–741.
Gamble CR, Huang Y, Tergas AI, et al. Quality of care and outcomes of patients with gynecologic malignancies treated at safety-net hospitals. JNCI Cancer Spectr. 2019;3(3):pkz039.
Liu FW, Randall LM, Tewari KS, Bristow RE. Racial disparities and patterns of ovarian cancer surgical care in California. Gynecol Oncol. 2014;132(1):221–226.
Cole AP, Nguyen DD, Meirkhanov A, et al. Association of care at minority-serving vs non-minority-serving hospitals with use of palliative care among racial/ethnic minorities with metastatic cancer in the United States. JAMA Netw Open. 2019;2(2):e187633.
Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575.
Giuliano AE, Ballman KV, McCall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318(10):918–926.
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–1461.
Donker M, van Tienhoven G, Straver ME, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer (EORTC 10981-22023 AMAROS): a randomised, multicentre, open-label, phase 3 non-inferiority trial. Lancet Oncol. 2014;15(12):1303–1310.
Rutgers EJ, Donker M, Poncet C, et al. Radiotherapy or surgery of the axilla after a positive sentinel node in breast cancer patients: 10 year follow up results of the EORTC AMAROS trial (EORTC 10981/22023). Paper presented at the San Antonio Breast Cancer Symposium: 6 December 2018; San Antonio, TX.
Yao K, Liederbach E, Pesce C, Wang CH, Winchester DJ. Impact of the American College of Surgeons Oncology Group Z0011 randomized trial on the number of axillary nodes removed for patients with early-stage breast cancer. J Am Coll Surg. 2015;221(1):71–81.
Caudle AS, Hunt KK, Tucker SL, et al. American College of Surgeons Oncology Group (ACOSOG) Z0011: impact on surgeon practice patterns. Ann Surg Oncol. 2012;19(10):3144–3151.
Caudle AS, Bedrosian I, Milton DR, et al. Use of sentinel lymph node dissection after neoadjuvant chemotherapy in patients with node-positive breast cancer at diagnosis: practice patterns of American Society of Breast Surgeons members. Ann Surg Oncol. 2017;24(10):2925–2934.
Srour MK, Tseng J, Luu M, Alban RF, Giuliano AE, Chung A. Patterns in the use of axillary operations for patients with node-positive breast cancer after neoadjuvant chemotherapy: a National Cancer Database (NCDB) analysis. Ann Surg Oncol. 2019;26(10):3305–3311.
Grossmith S, Nguyen A, Hu J, et al. Multidisciplinary management of the axilla in patients with cT1-T2 N0 breast cancer undergoing primary mastectomy: results from a prospective single-institution series. Ann Surg Oncol. 2018;25(12):3527–3534.
Weiss A, Lin H, Babiera GV, et al. Evolution in practice patterns of axillary management following mastectomy in patients with 1–2 positive sentinel nodes. Breast Cancer Res Treat. 2019;176(2):435–444.
National Comprehensive Cancer Network. Breast Cancer (Version 4.2020). https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 20 Jun 2020.
Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690.
Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726.
Chen MS Jr, Lara PN, Dang JH, Paterniti DA, Kelly K. Twenty years post-NIH Revitalization Act: enhancing minority participation in clinical trials (EMPaCT): laying the groundwork for improving minority clinical trial accrual: renewing the case for enhancing minority participation in cancer clinical trials. Cancer. 2014;120 Suppl 7(0 7):1091–1096.
Mitchell KW, Carey LA, Peppercorn J. Reporting of race and ethnicity in breast cancer research: room for improvement. Breast Cancer Res Treat. 2009;118(3):511–517. https://doi.org/10.1007/s10549-009-0411-4.
Albain KS, Unger JM, Crowley JJ, Coltman CA Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101(14):984–992. https://doi.org/10.1093/jnci/djp175.
Spain P, Teixeira-Poit S, Halpern MT, et al. The National Cancer Institute Community Cancer Centers Program (NCCCP): sustaining quality and reducing disparities in guideline-concordant breast and colon cancer care. Oncologist. 2017;22(8):910–917. https://doi.org/10.1634/theoncologist.2016-0252.
Commission on Cancer. Cancer Program Standards: Ensuring Patient-Centered Care, 2016 edition. https://www.facs.org/-/media/files/quality-programs/cancer/coc/2016-coc-standards-manual_interactive-pdf.ashx. Accessed 3 Jun 2020.
Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY. Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27(25):4177–4181.
Bensenhaver J, Winchester DP. Surgical leadership and standardization of multidisciplinary breast cancer care: the evolution of the National Accreditation Program for Breast Centers. Surg Oncol Clin N Am. 2014;23(3):609–616.
Knutson AC, McNamara EJ, McKellar DP, Kaufman CS, Winchester DP. The role of the American College of Surgeons’ cancer program accreditation in influencing oncologic outcomes. J Surg Oncol. 2014;110(5):611–615.
Kantor O, Wang CH, Yao K. Regional variation in performance for commission on cancer breast quality measures and impact on overall survival. Ann Surg Oncol. 2018;25(10):3069–3075.
Miller ME, Bleicher RJ, Kaufman CS, et al. Impact of breast center accreditation on compliance with breast quality performance measures at commission on cancer-accredited centers. Ann Surg Oncol. 2019;26(5):1202–1211.
Joynt KE, Orav EJ, Jha AK. Thirty-day readmission rates for Medicare beneficiaries by race and site of care. JAMA. 2011;305(7):675–681.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Olga Kantor, Cecilia Chang, Katharine Yao, Judy Boughey, Christina Roland, Amanda B. Francescatti, Sarah Blair, Diana Dickson Witmer, Kelly K. Hunt, Heidi Nelson, Anna Weiss, and Tawakalitu Oseni have no relevant financial disclosures to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kantor, O., Chang, C., Yao, K. et al. Uptake of Breast Cancer Clinical Trials at Minority Serving Cancer Centers. Ann Surg Oncol 28, 4995–5004 (2021). https://doi.org/10.1245/s10434-020-09533-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-09533-z