Abstract
Introduction
Oncotype DX® recurrence score (RS) is well-recognized for guiding decision making in adjuvant chemotherapy; however, the predictive capability of this genomic assay in determining axillary response to neoadjuvant chemotherapy (NCT) has not been established.
Methods
Using the National Cancer Data Base (NCDB), we identified patients diagnosed with T1-T2, clinically N1/N2, estrogen receptor-positive/human epidermal growth factor receptor 2-negative (ER +/HER2 −) invasive ductal carcinoma of the breast between 2010 and 2015. Patients with an Oncotype DX® RS who received NCT were included. RS was defined as low (< 18), intermediate (18–30), or high (> 30). Unadjusted and adjusted analyses were performed to determine the association between axillary pathologic complete response (pCR) and RS.
Results
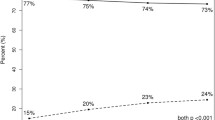
This study included a total of 158 women. RS was low in 56 (35.4%) patients, intermediate in 62 (39.2%) patients, and high in 40 (25.3%) patients. The majority of patients presented with clinical N1 disease (89.2%). Axillary pCR was achieved in 23 (14.6%) patients. When stratifying patients with axillary pCR by RS, 11 (47.8%) patients had a high RS, 6 (26.1%) patients had an intermediate RS, and 6 (26.1%) patients had a low RS. Comparing cohorts by RS, 27.5% of patients with high RS tumors had an axillary pCR, compared with only 9.7% in the intermediate RS group, and 10.7% in the low RS group (p = 0.0268).
Conclusion
Our findings demonstrate that Oncotype DX® RS is an independent predictor of axillary pCR in patients with ER +/HER2 − breast cancers receiving NCT. A greater proportion of patients with a high RS achieved axillary pCR. These results support Oncotype DX® as a tool to improve clinical decision making in axillary management.

Similar content being viewed by others
References
McCartan D, Gemignani ML. Current Management of the Axilla. Clin Obstet Gynecol. 2016;59(4):743–755. https://doi.org/10.1097/grf.0000000000000232
Rao R. The Evolution of Axillary Staging in Breast Cancer. Sci Med. 2015;112(5):385–388.
Veronesi U, Paganelli G, Viale G, et al. A randomized comparison of sentinel-node biopsy with routine axillary dissection in breast cancer. N Engl J Med. 2003;349(6):546–553. https://doi.org/10.1056/nejmoa012782
Lucci A, McCall LM, Beitsch PD, et al. Surgical complications associated with sentinel lymph node dissection (SLND) plus axillary lymph node dissection compared with SLND alone in the American College of Surgeons Oncology Group trial Z0011. J Clin Oncol. 2007;25(24):3657–3663. https://doi.org/10.1200/jco.2006.07.4062
Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: The ALMANAC trial. J Natl Cancer Inst. 2006;98(9):599–609. https://doi.org/10.1093/jnci/djj158
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (alliance) clinical trial. JAMA. 2013;310(14):1455–1461. https://doi.org/10.1001/jama.2013.278932
Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: The SN FNAC study. J Clin Oncol. 2015;33(3):258–263. https://doi.org/10.1200/jco.2014.55.7827
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609-618. https://doi.org/10.1016/s1470-2045(13)70166-9
Fayanju OM, Park KU, Lucci A. Molecular Genomic Testing for Breast Cancer: Utility for Surgeons. Ann Surg Oncol. 2018;25(2):512-519. https://doi.org/10.1245/s10434-017-6254-z
Paik S, Shak S, Tang g, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351:2817–2826. https://doi.org/10.1056/nejmoa041588
Iglehart JD, Valero MG. Commentary on The role of the breast cancer surgeon in personalized cancer care: clinical utility of the 21-gene assay. Am J Surg. 2012;203(6):759–760. https://doi.org/10.1016/j.amjsurg.2011.09.030
Mamounas EP, Russell CA, Lau A, Turner MP, Albain KS. Clinical relevance of the 21-gene Recurrence Score® assay in treatment decisions for patients with node-positive breast cancer in the genomic era. npj Breast Cancer. 2018;4(1). https://doi.org/10.1038/s41523-018-0082-6
Pease AM, Riba LA, Gruner RA, Tung NM, James TA. Oncotype DX ® Recurrence Score as a Predictor of Response to Neoadjuvant Chemotherapy. Ann Surg Oncol. 2019;26(2):366–371. https://doi.org/10.1245/s10434-018-07107-8
Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and resection of clipped node decreases the false-negative rate of sentinel lymph node surgery in patients presenting with node-positive breast cancer (T0-T4, N1-N2) who receive neoadjuvant chemotherapy: results from ACOSOG Z1071 (Alliance). Ann Surg. 2016;263(4):802–807. https://doi.org/10.1097/sla.0000000000001375
Palmer JAV, Flippo-Morton T, Walsh KK, et al. Application of ACOSOG Z1071: Effect of Results on Patient Care and Surgical Decision-Making. Clin Breast Cancer. 2018;18(4):270–275. https://doi.org/10.1016/j.clbc.2017.10.006
Kantor O, Sipsy LMN, Yao K, James TA. A Predictive Model for Axillary Node Pathologic Complete Response after Neoadjuvant Chemotherapy for Breast Cancer. Ann Surg Oncol. 2018;25(5):1304–1311. https://doi.org/10.1245/s10434-018-6345-5
Choi HJ, Ryu JM, Kim I, et al. Prediction of axillary pathologic response with breast pathologic complete response after neoadjuvant chemotherapy. Breast Cancer Research and Treatment. 2019;176(3):591–596. https://doi.org/10.1007/s10549-019-05214-y
Schipper RJ, Moossdorff M, Nelemans PJ, et al. A model to predict pathologic complete response of axillary lymph nodes to neoadjuvant chemo(immuno)therapy in patients with clinically node-positive breast cancer. Clin Breast Cancer. 2014;14(5):315–322. https://doi.org/10.1016/j.clbc.2013.12.015
Kim JY, Park HS, Kim S, Ryu J, Park S, Kim S Il. Prognostic nomogram for prediction of axillary pathologic complete response after neoadjuvant chemotherapy in cytologically proven node-positive breast cancer. Med (United States). 2015;94(43):1–9. https://doi.org/10.1097/md.0000000000001720
Lee SB, Kim J, Sohn G, et al. A nomogram for predicting the Oncotype DX recurrence score in women with T1-3N0-1miM0 hormone receptor-positive, human epidermal growth factor-2 (HER2)-negative breast cancer. Cancer Research and Treatment. 2019;51(3):1073–1085. https://doi.org/10.4143/crt.2018.357
Orucevic A, Bell JL, McNabb AP, Heidel RE. Oncotype DX breast cancer recurrence score can be predicted with a novel nomogram using clinicopathologic data. Breast Cancer Res Treat. 2017;163(1):51–61. https://doi.org/10.1007/s10549-017-4170-3
Yoo SH, Kim TY, Kim M, et al. Development of a Nomogram to Predict the Recurrence Score of 21-Gene Prediction Assay in Hormone Receptor–Positive Early Breast Cancer. Clin Breast Cancer. 2019;20(2):98-107.e1. https://doi.org/10.1016/j.clbc.2019.07.010
Orucevic A, Bell JL, King M, McNabb AP, Heidel RE. Nomogram update based on TAILORx clinical trial results: Oncotype DX breast cancer recurrence score can be predicted using clinicopathologic data. Breast. 2019;46:116–125. https://doi.org/10.1016/j.breast.2019.05.006
Soran A, Tane K, Sezgin E, Bhargava R. The Correlation of Magee EquationsTM and Oncotype DX® Recurrence Score From Core Needle Biopsy Tissues in Predicting Response to Neoadjuvant Chemotherapy in ER + and HER2- Breast Cancer. Eur J Breast Heal. 2020;16(2):117–123. https://doi.org/10.5152/ejbh.2020.5338
National Cancer Institute. Tamoxifen citrate, letrozole, anastrozole, or exemestane with or without chemotherapy in treating patients with invasive RxPONDER breast cancer. ClinicalTrials.gov identifider: NCT01272037. Available at: https://clinicaltrials.gov/ct2/show/NCT01272037. Accessed 5 Mar 2020.
Baron P, Beitsch P, Boselli D, et al. Impact of Tumor Size on Probability of Pathologic Complete Response After Neoadjuvant Chemotherapy. Ann Surg Oncol. 2016;23(5):1522–1529. https://doi.org/10.1245/s10434-015-5030-1
Fayanju OM, Ren Y, Thomas SM, et al. The clinical significance of breast-only and node-only pathologic complete response (PCR) after neoadjuvant chemotherapy (NACT): A review of 20,000 breast cancer patients in the National Cancer Data Base (NCDB). Ann Surg. 2018;268(4):591–601. https://doi.org/10.1097/sla.0000000000002953
Riba LA, Russell T, Alapati A, Davis RB, James TA. Characterizing response to neoadjuvant chemotherapy in invasive lobular breast carcinoma. J Surg Res. 2019;233:436–443. https://doi.org/10.1016/j.jss.2018.08.011
Tubiana-Hulin M, Stevens D, Lasry S, et al. Response to neoadjuvant chemotherapy in lobular and ductal breast carcinomas: a retrospective study on 860 patients from one institution. Ann Oncol. 2006;17(8):1228–1233. https://doi.org/10.1093/annonc/mdl114
Cristofanilli M, Gonzalez-Angulo A, Sneige N, et al. Invasive lobular carcinoma classic type: Response to primary chemotherapy and survival outcomes. J Clin Oncol. 2005;23(1):41–48. https://doi.org/10.1200/jco.2005.03.111
Cocquyt VF, Blondeel PN, Depyperet HT, et al. Different responses to preoperative chemotherapy for invasive lobular and invasive ductal breast carcinoma. Eur J Surg Oncol. 2003;29(4):361–367. https://doi.org/10.1053/ejso.2002.1404
Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–121. https://doi.org/10.1056/nejmoa1804710
National Comprehensive Cancer Network. NCCN Guidelines Breast Cancer Version 6.2020. 2020. Available at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
Acknowledgment
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Ted James is a scientific consultant for Perimeter Medical. Jaime A. Pardo, Betty Fan, Alessandra Mele, Stephanie Serres, Monica G. Valero, Isha Emhoff, and Amulya Alapati have no disclosures to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pardo, J.A., Fan, B., Mele, A. et al. The Role of Oncotype DX® Recurrence Score in Predicting Axillary Response After Neoadjuvant Chemotherapy in Breast Cancer. Ann Surg Oncol 28, 1320–1325 (2021). https://doi.org/10.1245/s10434-020-09382-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-09382-w