Abstract
Background
Cutaneous melanoma and distant organ metastasis has varying outcomes. Considering all prognostic indicators in a prediction model might assist in selecting cases who could benefit from a personalized therapy strategy.
Objective
This study aimed to develop and validate a prognostic model for patients with metastatic melanoma.
Methods
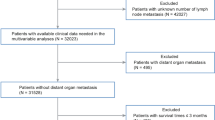
A total of 1535 cases diagnosed with metastatic cutaneous melanoma (stage IV) were identified from the Surveillance, Epidemiology, and End Results database. Patients were randomly divided into the training (n = 1023) and validation (n = 512) cohorts. A prognostic nomogram was established based predominantly on results from the competing-risk regression model for predicting cancer-specific death (CSD). The area under the time-dependent receiver operating characteristic curve (AUC), calibration curves, and decision curve analyses (DCAs) were used to evaluate the nomogram.
Results
No significant differences were observed in the clinical characteristics between the training and validation cohorts. In the training cohort, patient-, tumor-, and treatment-related predictors of CSD for metastatic melanoma included age, sex, race, marital status, insurance, American Joint Committee on Cancer T and N stage, number of metastatic organs, surgical treatment, and chemotherapy. All these factors were used for nomogram construction. The time-dependent AUC values of the training and validation cohorts suggested a favorable performance and discrimination of the nomogram. The 6-, 12-, and 18-month AUC values were 0.706, 0.700, and 0.706 in the training cohort, and 0.702, 0.670, and 0.656 in the validation cohort, respectively. The calibration curves for the probability of death at 6, 12, and 18 months showed acceptable agreement between the values predicted by the nomogram and the observed outcomes in both cohorts. DCA curves showed good positive net benefits in the prognostic model among most of the threshold probabilities at different time points (death at 6, 12, and 18 months). Based on the total nomogram scores of each case, all patients were divided into the low-risk (n = 511), intermediate-risk (n = 512), and high-risk (n = 512) groups, and the risk classification could identify cases with a high risk of death in both cohorts.
Conclusions
A predictive nomogram and a corresponding risk classification system for CSD in patients with metastatic melanoma were developed in this study, which may assist in patient counseling and in guiding clinical decision making for cases with metastatic melanoma.





Similar content being viewed by others
References
Geller AC, Clapp RW, Sober AJ, et al. Melanoma epidemic: an analysis of six decades of data from the Connecticut Tumor Registry. Journal of clinical oncology. 2013;31(33):4172–4178.
Keung EZ, Gershenwald JE. Clinicopathological Features, Staging, and Current Approaches to Treatment in High-Risk Resectable Melanoma. Journal of the National Cancer Institute. 2020;112(9):875–885.
O’Neill CH, McMasters KM, Egger ME. Role of Surgery in Stage IV Melanoma. Surgical oncology clinics of North America. 2020;29(3):485–495.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: a cancer journal for clinicians. 2018;68(1):7–30.
Gardner LJ, Ward M, Andtbacka RHI, et al. Risk factors for development of melanoma brain metastasis and disease progression: a single-center retrospective analysis. Melanoma research. 2017;27(5):477–484.
Viehof J, Livingstone E, Loscha E, et al. Prognostic factors for pulmonary metastasectomy in malignant melanoma: size matters. European journal of cardio-thoracic surgery. 2019;56(6):1104–1109.
Barnhill R, van Dam PJ, Vermeulen P, et al. Replacement and desmoplastic histopathological growth patterns in cutaneous melanoma liver metastases: frequency, characteristics, and robust prognostic value. The journal of pathology Clinical research. 2020;6(3):195–206.
Wu H, Xia L, Jia D, et al. PD-L1(+) regulatory B cells act as a T cell suppressor in a PD-L1-dependent manner in melanoma patients with bone metastasis. Molecular immunology. 2020;119:83–91.
Yoshida S, Fujimura T, Kambayashi Y, Amagai R, Hashimoto A, Aiba S. Successful Treatment of Multiple Metastatic Melanoma with Nivolumab, Ipilimumab plus Denosumab Combined Therapy. Case reports in oncology. 2019;12(3):829–833.
Ollila DW. Complete metastasectomy in patients with stage IV metastatic melanoma. The Lancet Oncology. 2006;7(11):919–924.
Faries MB, Mozzillo N, Kashani-Sabet M, et al. Long-Term Survival after Complete Surgical Resection and Adjuvant Immunotherapy for Distant Melanoma Metastases. Annals of surgical oncology. 2017;24(13):3991–4000.
Lasithiotakis K, Zoras O. Metastasectomy in cutaneous melanoma. European journal of surgical oncology. 2017;43(3):572–580.
Geukes Foppen MH, Brandsma D, Blank CU, van Thienen JV, Haanen JB, Boogerd W. Targeted treatment and immunotherapy in leptomeningeal metastases from melanoma. Annals of oncology. 2016;27(6):1138–1142.
Harries M, Malvehy J, Lebbe C, et al. Treatment patterns of advanced malignant melanoma (stage III-IV): a review of current standards in Europe. European journal of cancer. 2016;60:179–189.
Riaz N, Havel JJ, Makarov V, et al. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell. 2017;171(4):934–949.e916.
Grob JJ, Garbe C, Ascierto P, Larkin J, Dummer R, Schadendorf D. Adjuvant melanoma therapy with new drugs: should physicians continue to focus on metastatic disease or use it earlier in primary melanoma? The Lancet. Oncology. 2018;19(12):e720–e725.
Goldberg SB, Gettinger SN, Mahajan A, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. The Lancet Oncology. 2016;17(7):976–983.
Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nature reviews Clinical oncology. 2017;14(8):463–482.
Nosrati A, Yu WY, McGuire J, et al. Outcomes and Risk Factors in Patients with Multiple Primary Melanomas. The Journal of investigative dermatology. 2019;139(1):195–201.
Kim JE, Chung BY, Sim CY, Park AY, Lee JS, Whang KU, et al.. Clinicopathologic Features and Prognostic Factors of Primary Cutaneous Melanoma: a Multicenter Study in Korea. Journal of Korean medical science. 2019;34(16):e126.
Richetta AG, Valentini V, Marraffa F, et al. Metastases risk in thin cutaneous melanoma: prognostic value of clinical-pathologic characteristics and mutation profile. Oncotarget. 2018;9(63):32173–32181.
Liu H, Xu YB, Guo CC, Li MX, Ji JL, Dong RR, et al. Predictive value of a nomogram for melanomas with brain metastases at initial diagnosis. Cancer medicine. 2019;8(18):7577–7585.
de Wreede LC, Fiocco M, Putter H. The mstate package for estimation and prediction in non- and semi-parametric multi-state and competing risks models. Computer methods and programs in biomedicine. 2010;99(3):261–274.
Jaddoe VW, de Jonge LL, Hofman A, Franco OH, Steegers EA, Gaillard R. First trimester fetal growth restriction and cardiovascular risk factors in school age children: population based cohort study. BMJ. 2014;348:g14.
Kernan WN, Viscoli CM, Brass LM, et al. Phenylpropanolamine and the risk of hemorrhagic stroke. The New England journal of medicine. 2000;343(25):1826–1832.
Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. The New England journal of medicine. 2011;364(3):226–235.
Heagerty PJ, Lumley T, Pepe MS. Time-dependent ROC curves for censored survival data and a diagnostic marker. Biometrics. 2000;56(2):337–344.
Vickers AJ, Elkin EB. Decision curve analysis: a novel method for evaluating prediction models. Medical decision making. 2006;26(6):565–574.
Kerr KF, Brown MD, Zhu K, Janes H. Assessing the Clinical Impact of Risk Prediction Models With Decision Curves: Guidance for Correct Interpretation and Appropriate Use. Journal of clinical oncology. 2016;34(21):2534–2540.
Tang X, Zhou X, Li Y, et al. A Novel Nomogram and Risk Classification System Predicting the Cancer-Specific Survival of Patients with Initially Diagnosed Metastatic Esophageal Cancer: A SEER-Based Study. Annals of surgical oncology. 2019;26(2):321–328.
Wang Y, Pang Z, Chen X, et al. Survival nomogram for patients with initially diagnosed metastatic non-small-cell lung cancer: a SEER-based study. Future oncology. 2019;15(29):3395–3409.
Yuan TA, Lu Y, Edwards K, Jakowatz J, Meyskens FL, Liu-Smith F. Race-, Age-, and Anatomic Site-Specific Gender Differences in Cutaneous Melanoma Suggest Differential Mechanisms of Early- and Late-Onset Melanoma. Int J Environ Res Public Health. 2019;16(6):908.
Haque W, Verma V, Butler EB, Teh BS. Racial and Socioeconomic Disparities in the Delivery of Immunotherapy for Metastatic Melanoma in the United States. Journal of immunotherapy. 2019;42(6):228–235.
Bellenghi M, Puglisi R, Pontecorvi G, De Feo A, Carè A. Sex and Gender Disparities in Melanoma. Cancers (Basel). 2020;12(7):1819.
Tripathi R, Archibald LK, Mazmudar RS, et al. Racial differences in time to treatment for melanoma. Journal of the American Academy of Dermatology. 2020;83(3):854–859.
Rajeshuni N, Zubair T, Ludwig CA, Moshfeghi DM, Mruthyunjaya P. Evaluation of Racial, Ethnic, and Socioeconomic Associations With Treatment and Survival in Uveal Melanoma, 2004-2014. JAMA ophthalmology. 2020;138(8):876–884.
Abdel-Rahman O. Prognostic impact of socioeconomic status among patients with malignant melanoma of the skin: a population-based study. The Journal of dermatological treatment. 2020;31(6):571–575.
Maas JA, Monreal AJ, Diaz EL, Castro G, Rodriguez de la Vega P, Varella M. Marital Status and Survival in Patients Diagnosed with Melanoma. Dermatology research and practice. 2020;2020:2485401.
Loya A, Ayaz T, Weng CY. Impact of Marital Status on Survival in Patients with Ocular and Periocular Malignancies: A Retrospective Analysis of 3159 Patients from the SEER Database. Clinical ophthalmology. 2020;14:1127–1133.
Rauwerdink DJW, Molina G, Frederick DT, et al. Mixed Response to Immunotherapy in Patients with Metastatic Melanoma. Annals of surgical oncology. 2020;27(9):3488–3497.
Tyrell R, Antia C, Stanley S, Deutsch GB. Surgical resection of metastatic melanoma in the era of immunotherapy and targeted therapy. Melanoma management. 2017;4(1):61–68.
Kurtz J, Beasley GM, Agnese D, et al. Surveillance strategies in the follow-up of melanoma patients: too much or not enough? The Journal of surgical research. 2017;214:32–37.
Baranowski MLH, Yeung H, Chen SC, Gillespie TW, Goodman M. Factors associated with time to surgery in melanoma: an analysis of the National Cancer Database. The British journal of dermatology. 2019;81(4):908–916.
Yang J, Pan Z, Zhao F, et al. A nomogram for predicting survival in patients with nodular melanoma: a population-based study. Medicine. 2019;98(24):e16059.
Verver D, van Klaveren D, Franke V, et al. Development and validation of a nomogram to predict recurrence and melanoma-specific mortality in patients with negative sentinel lymph nodes. The British journal of surgery. 2019;106(3):217–225.
Funding
No sources of funding were used in the preparation of this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Disclosure
Wei Li, Yang Xiao, Xuewen Xu, and Yange Zhang have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, W., Xiao, Y., Xu, X. et al. A Novel Nomogram and Risk Classification System Predicting the Cancer-Specific Mortality of Patients with Initially Diagnosed Metastatic Cutaneous Melanoma. Ann Surg Oncol 28, 3490–3500 (2021). https://doi.org/10.1245/s10434-020-09341-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-09341-5