Abstract
Background
Administration of dexamethasone to mitigate postoperative nausea and vomiting has been suggested to improve short- and long-term outcomes after pancreatic ductal adenocarcinoma (PDAC) resection. This study aimed primarily to evaluate these hypotheses in a contemporary patient cohort treated with multimodality therapy.
Methods
The clinicopathologic and perioperative characteristics of consecutive resected PDAC patients (July 2011 to October 2018) were analyzed from a prospectively maintained database. Intraoperative administration of dexamethasone (4–10 mg) was retrospectively abstracted from the electronic medical record.
Results
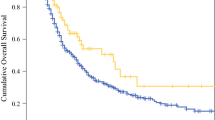
The majority of 373 patients (59.8%) received intraoperative dexamethasone. Most of these patients underwent neoadjuvant therapy (75.3%), were potentially resectable at presentation (69.7%), and underwent pancreaticoduodenectomy (79.9%). Women were more likely to receive dexamethasone than men (69.9 vs 30.1%; p < 0.001). The cohorts were otherwise clinically similar. Intraoperative dexamethasone was not associated with differences in postoperative major complications (PMCs) (21.1 vs 19.3%; p = 0.68), postoperative pancreatic fistulas (6.3 vs 6.7%; p = 0.88), or composite infectious complications (28.7 vs 24.7%; p = 0.39). Dexamethasone was not associated with any improvement in median recurrence-free survival (RFS) (17 vs 17 months; p = 0.99) or overall survival (OS) (46 vs 43 months; p = 0.90). After adjustment for clinical factors including margin status, clinical classification, tumor size, and dexamethasone, the only factors independently associated with OS were pathologic node-positivity (hazard ratio [HR], 1.80, 95% confidence interval [CI], 1.32–2.47), perineural invasion (HR, 2.02; 95% CI, 1.23–3.31), multimodality therapy (HR, 0.30; 95% CI, 0.13–0.70), and PMCs (HR, 1.64; 95% CI, 1.17–2.29) (all p < 0.006).
Conclusions
Dexamethasone failed to demonstrate any protective advantage in terms of mitigating short-term PMCs or infectious complications, or to confer any long-term survival benefit. Tumor biology, multimodality therapy, and PMCs remain the main prognostic factors after PDAC resection.

Similar content being viewed by others
References
Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21.
Janssen QP, Buettner S, Suker M, et al. Neoadjuvant FOLFIRINOX in patients with borderline resectable pancreatic cancer: a systematic review and patient-level meta-analysis. J Natl Cancer Inst. 2019;111:782–94.
Kim MP, Katz MHG. Perioperative therapy for borderline resectable pancreatic cancer: what and when? Ann Surg Oncol. 2019;26:1596–7.
Schwarz L, Katz MH. Diagnosis and management of borderline resectable pancreatic adenocarcinoma. Hematol Oncol Clin North Am. 2015;29:727–40.
Katz MH, Crane CH, Varadhachary G. Management of borderline resectable pancreatic cancer. Semin Radiat Oncol. 2014;24:105–12.
Cooper AB, Tzeng CW, Katz MH. Treatment of borderline resectable pancreatic cancer. Curr Treat Options Oncol. 2013;14:293–310.
Blajchman MA. Immunomodulation and blood transfusion. Am J Ther. 2002;9:389–95.
Call TR, Pace NL, Thorup DB, et al. Factors associated with improved survival after resection of pancreatic adenocarcinoma: a multivariable model. Anesthesiology. 2015;122:317–24.
Lassen K, Coolsen MM, Slim K, et al. Guidelines for perioperative care for pancreaticoduodenectomy: Enhanced Recovery After Surgery (ERAS(R)) Society recommendations. Clin Nutr. 2012;31:817–30.
Lopez-Aguiar AG, Ethun CG, Pawlik TM, et al. Association of perioperative transfusion with recurrence and survival after resection of distal cholangiocarcinoma: a 10-institution study from the US Extrahepatic Biliary Malignancy Consortium. Ann Surg Oncol. 2019;26:1814–23.
Zimmitti G, Soliz J, Aloia TA, et al. Positive impact of epidural analgesia on oncologic outcomes in patients undergoing resection of colorectal liver metastases. Ann Surg Oncol. 2016;23:1003–11.
Sandini M, Ruscic KJ, Ferrone CR, et al. Intraoperative dexamethasone decreases infectious complications after pancreaticoduodenectomy and is associated with long-term survival in pancreatic cancer. Ann Surg Oncol. 2018;25:4020–6.
Leahy KM, Ornberg RL, Wang Y, Zweifel BS, Koki AT, Masferrer JL. Cyclooxygenase-2 inhibition by celecoxib reduces proliferation and induces apoptosis in angiogenic endothelial cells in vivo. Cancer Res. 2002;62:625–31.
Tsuchiya Y, Sawada S, Yoshioka I, et al. Increased surgical stress promotes tumor metastasis. Surgery. 2003;133:547–55.
Cata JP, Jones J, Sepesi B, et al. Lack of association between dexamethasone and long-term survival after non-small cell lung cancer surgery. J Cardiothorac Vasc Anesth. 2016;30:930–5.
Gan TJ, Diemunsch P, Habib AS, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analgesia. 2014;118:85–113.
Schwarz L, Bruno M, Parker NH, et al. Active surveillance for adverse events within 90 days: the standard for reporting surgical outcomes after pancreatectomy. Ann Surg Oncol. 2015;22:3522–9.
Denbo JW, Bruno M, Dewhurst W, et al. Risk-stratified clinical pathways decrease the duration of hospitalization and costs of perioperative care after pancreatectomy. Surgery. 2018;164:424–31.
Strasberg SM, Linehan DC, Hawkins WG. The accordion severity grading system of surgical complications. Ann Surg. 2009;250:177–86.
Wente MN, Bassi C, Dervenis C, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142:761–8.
Bassi C, Marchegiani G, Dervenis C, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 2017;161:584–91.
Kwon S, Thompson R, Dellinger P, Yanez D, Farrohki E, Flum D. Importance of perioperative glycemic control in general surgery: a report from the Surgical Care and Outcomes Assessment Program. Ann Surg. 2013;257:8–14.
Bartlett R, Hartle AJ. Routine use of dexamethasone for postoperative nausea and vomiting: the case against. Anaesthesia. 2013;68:892–96.
De Oliveira GS Jr, McCarthy R, Turan A, Schink JC, Fitzgerald PC, Sessler DI. Is dexamethasone associated with recurrence of ovarian cancer? Anesth Analgesia. 2014;118:1213–8.
Cloyd JM, Heh V, Pawlik TM, et al. Neoadjuvant therapy for resectable and borderline resectable pancreatic cancer: a meta-analysis of randomized controlled trials. J Clin Med. 2020;9(4):1129.
Cloyd JM, Wang H, Egger ME, et al. Association of clinical factors with a major pathologic response following preoperative therapy for pancreatic ductal adenocarcinoma. JAMA Surg. 2017;152:1048–56.
Motoi F, Kosuge T, Ueno H, et al. Randomized phase II/III trial of neoadjuvant chemotherapy with gemcitabine and S-1 versus upfront surgery for resectable pancreatic cancer (Prep-02/JSAP05). Jpn J Clin Oncol. 2019;49:190–4.
Motoi F, Satoi S, Honda G, et al. A single-arm, phase II trial of neoadjuvant gemcitabine and S1 in patients with resectable and borderline resectable pancreatic adenocarcinoma: PREP-01 study. J Gastroenterol. 2019;54:194–203.
Tzeng CW, Tran Cao HS, Lee JE, et al. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg. 2014;18(1):16–25.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Newhook, T.E., Soliz, J.M., Prakash, L.R. et al. Impact of Intraoperative Dexamethasone on Surgical and Oncologic Outcomes for Patients with Resected Pancreatic Ductal Adenocarcinoma. Ann Surg Oncol 28, 1563–1569 (2021). https://doi.org/10.1245/s10434-020-09013-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-09013-4