Abstract
Background
Surgical resection of hepatic metastases remains the only potentially curative treatment option for patients with colorectal liver metastases (CRLM). Widely adopted prognostic tools may oversimplify the impact of model parameters relative to long-term outcomes.
Methods
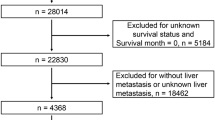
Patients with CRLM who underwent a hepatectomy between 2001 and 2018 were identified in an international, multi-institutional database. Bootstrap resampling methodology used in tandem with multivariable mixed-effects logistic regression analysis was applied to construct a prediction model that was validated and compared with scores proposed by Fong and Vauthey.
Results
Among 1406 patients who underwent hepatic resection of CRLM, 842 (59.9%) had recurrence. The full model (based on age, sex, primary tumor location, T stage, receipt of chemotherapy before hepatectomy, lymph node metastases, number of metastatic lesions in the liver, size of the largest hepatic metastases, carcinoembryonic antigen [CEA] level and KRAS status) had good discriminative ability to predict 1-year (area under the receiver operating curve [AUC], 0.693; 95% confidence interval [CI], 0.684–0.704), 3-year (AUC, 0.669; 95% CI, 0.661–0.677), and 5-year (AUC, 0.669; 95% CI, 0.661–0.679) risk of recurrence. Studies analyzing validation cohorts demonstrated similar model performance, with excellent model accuracy. In contrast, the AUCs for the Fong and Vauthey scores to predict 1-year recurrence were only 0.527 (95% CI, 0.514–0.538) and 0.525 (95% CI, 0.514–0.533), respectively. Similar trends were noted for 3- and 5-year recurrence.
Conclusion
The proposed clinical score, derived via machine learning, which included clinical characteristics and morphologic data, as well as information on KRAS status, accurately predicted recurrence after CRLM resection with good discrimination and prognostic ability.



Similar content being viewed by others
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
White A, Joseph D, Rim SH, Johnson CJ, Coleman MP, Allemani C. Colon cancer survival in the United States by race and stage (2001–2009): findings from the CONCORD-2 study. Cancer. 2017;123(Suppl 24):5014–36.
Engstrand J, Nilsson H, Stromberg C, Jonas E, Freedman J. Colorectal cancer liver metastases: a population-based study on incidence, management, and survival. BMC Cancer. 2018;18:78.
Sahara K, Tsilimigras DI, Paredes AZ, et al. Development and validation of a real-time mortality risk calculator before, during, and after hepatectomy: an analysis of the ACS NSQIP database. HPB Oxford. 2019.
Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: Analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–18.
Brudvik KW, Jones RP, Giuliante F, et al. RAS mutation clinical risk score to predict survival after resection of colorectal liver metastases. Ann Surg. 2019;269:120–6.
Mann CD, Metcalfe MS, Leopardi LN, Maddern GJ. The clinical risk score: emerging as a reliable preoperative prognostic index in hepatectomy for colorectal metastases. Arch Surg. 2004;139:1168–72.
Beamish P, Lemke M, Li J, et al. Validation of clinical risk score for colorectal liver metastases resected in a contemporary multicenter cohort. HPB Oxford. 2017;19:675–81.
Ayez N, Lalmahomed ZS, van der Pool AE, et al. Is the clinical risk score for patients with colorectal liver metastases still useable in the era of effective neoadjuvant chemotherapy? Ann Surg Oncol. 2011;18:2757–63.
Balachandran VP, Arora A, Gonen M, et al. A validated prognostic multigene expression assay for overall survival in resected colorectal cancer liver metastases. Clin Cancer Res. 2016;22:2575–82.
Margonis GA, Kim Y, Spolverato G, et al. Association between specific mutations in KRAS codon 12 and colorectal liver metastasis. JAMA Surg. 2015;150:722–9.
Winter D. Colorectal liver metastasis resection outcomes defined by molecular biology. JAMA Surg. 2015;150:729.
Margonis GA, Buettner S, Andreatos N, et al. Association of BRAF mutations with survival and recurrence in surgically treated patients with metastatic colorectal liver cancer. JAMA Surg. 2018;153:e180996.
Tsilimigras DI, Ntanasis-Stathopoulos I, Bagante F, et al. Clinical significance and prognostic relevance of KRAS, BRAF, PI3K, and TP53 genetic mutation analysis for resectable and unresectable colorectal liver metastases: a systematic review of the current evidence. Surg Oncol. 2018;27:280–8.
Margonis GA, Spolverato G, Kim Y, Karagkounis G, Choti MA, Pawlik TM. Effect of KRAS mutation on long-term outcomes of patients undergoing hepatic resection for colorectal liver metastases. Ann Surg Oncol. 2015;22:4158–65.
Moons KG, Altman DG, Reitsma JB, et al. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med. 2015;162:W1–73.
Oussoultzoglou E, Rosso E, Fuchshuber P, et al. Perioperative carcinoembryonic antigen measurements to predict curability after liver resection for colorectal metastases: a prospective study. Arch Surg. 2008;143:1150–8; discussion 1158–9.
van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res. 2007;16:219–42.
Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8:206–13.
McCleary L. Using multiple imputation for analysis of incomplete data in clinical research. Nurs Res. 2002;51:339–43.
Breiman L. Bagging predictors. Mach Learn. 1996;24:123–40.
American Cancer Society. Global cancer facts and figures, 2018. Retrieved 15 February 2020 at https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/global-cancer-facts-and-figures/global-cancer-facts-and-figures-4th-edition.pdf.
Adam R, Kitano Y. Multidisciplinary approach of liver metastases from colorectal cancer. Ann Gastroenterol Surg. 2019;3:50–6.
Schreckenbach T, Malkomes P, Bechstein WO, Woeste G, Schnitzbauer AA, Ulrich F. The clinical relevance of the Fong and the Nordlinger scores in the era of effective neoadjuvant chemotherapy for colorectal liver metastasis. Surg Today. 2015;45:1527–34.
Sasaki K, Margonis GA, Wilson A, et al. Prognostic implication of KRAS status after hepatectomy for colorectal liver metastases varies according to primary colorectal tumor location. Ann Surg Oncol. 2016;23:3736–43.
Adam R, Delvart V, Pascal G, et al. Rescue surgery for unresectable colorectal liver metastases downstaged by chemotherapy: A model to predict long-term survival. Ann Surg. 2004;240:644–57.
Margonis GA, Sasaki K, Gholami S, et al. Genetic and morphological evaluation (GAME) score for patients with colorectal liver metastases. Br J Surg. 2018;105:1210–20.
Nordlinger B, Guiguet M, Vaillant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver: a prognostic scoring system to improve case selection, based on 1568 patients. Association Francaise de Chirurgie. Cancer. 1996;77:1254–62.
Konopke R, Kersting S, Distler M, et al. Prognostic factors and evaluation of a clinical score for predicting survival after resection of colorectal liver metastases. Liver Int. 2009;29:89–102.
Dupre A, Rehman A, Jones RP, et al. Validation of clinical prognostic scores for patients treated with curative-intent for recurrent colorectal liver metastases. J Surg Oncol. 2018;117:1330–6.
Li P, Stuart EA, Allison DB. Multiple imputation: a flexible tool for handling missing data. JAMA. 2015;314:1966–7.
Schomaker M, Heumann C. Bootstrap inference when using multiple imputation. Stat Med. 2018;37:2252–66.
Fakih MG. Metastatic colorectal cancer: current state and future directions. J Clin Oncol. 2015;33:1809–24.
Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist. 2008;13:51–64.
Harrison XA, Donaldson L, Correa-Cano ME, et al. A brief introduction to mixed effects modelling and multi-model inference in ecology. PeerJ. 2018;6:e4794.
Pavlou M, Ambler G, Seaman S, Omar RZ. A note on obtaining correct marginal predictions from a random intercepts model for binary outcomes. BMC Med Res Methodol. 2015;15:59.
Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ. 2009;338:b2393.
Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
There are no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Paredes, A.Z., Hyer, J.M., Tsilimigras, D.I. et al. A Novel Machine-Learning Approach to Predict Recurrence After Resection of Colorectal Liver Metastases. Ann Surg Oncol 27, 5139–5147 (2020). https://doi.org/10.1245/s10434-020-08991-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08991-9