Abstract
Introduction
Prediction models are useful to guide decision making. Our goal was to compare three published nomograms predicting axillary response to neoadjuvant chemotherapy (NAC), clinically node-positive breast cancer.
Methods
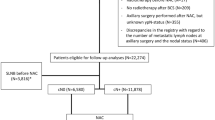
Patients with cT1–T4, cN1–N3 breast cancer treated with NAC and surgery from 2008 to 2019 were reviewed. The predicted probability of pathologic node-negative (ypN0) status was estimated for each nomogram. Area under the curve (AUC) was compared across models, overall and by biologic subtype.
Results
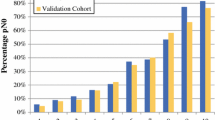
Of 581 patients, 253 (43.5%) were ypN0. ypN0 status varied by subtype: 23.9% for estrogen receptor-positive (ER+)/human epidermal growth factor receptor 2-negative (HER2−), 68.9% for HER2-positive (HER2+), and 47.2% for ER-negative (ER−)/HER2−. The three nomograms had similar AUC values (0.761–0.769; p = 0.80). The Mayo model-predicted probability was significantly lower (p < 0.001) than the observed probability of ypN0 status, while the MD Anderson Cancer Center (MDACC) 1- and 2-predicted probabilities were similar to the observed probability. At a predicted probability threshold of 50%, the Mayo model had the highest sensitivity (89.6%) for detecting ypN+ patients compared with MDACC models 1 and 2 (76.5%; p < 0.001). However, both MDACC models had higher specificity in identifying ypN0 status among HER2+ (81.7%) and ER−/HER2− (75.9–77.6%) patients compared with the Mayo model (59.5% and 43.1%; each p < 0.001). None of the models identified the ER+/HER2− patients with ypN0 status well at the ≥ 50% threshold (specificity 0–9.4%).
Conclusion
All three models predicting nodal response to NAC performed well overall with respect to discrimination, but differed with respect to calibration and performance at a 50% probability threshold. However, none of the models performed well at the 50% threshold for ER+/HER2− patients.


Similar content being viewed by others
References
Barron AU, Hoskin TL, Boughey JC. Predicting non-sentinel lymph node metastases in patients with a positive sentinel lymph node after neoadjuvant chemotherapy. Ann Surg Oncol. 2018;25(10):2867–74.
Kantor O, Sipsy LM, Yao K, James TA. A predictive model for axillary node pathologic complete response after neoadjuvant chemotherapy for breast cancer. Ann Surg Oncol. 2018;25(5):1304–11.
Vila J, Mittendorf EA, Farante G, et al. Nomograms for predicting axillary response to neoadjuvant chemotherapy in clinically node-positive patients with breast cancer. Ann Surg Oncol. 2016;23(11):3501–9.
Schipper RJ, Moossdorff M, Nelemans PJ, et al. A model to predict pathologic complete response of axillary lymph nodes to neoadjuvant chemo(immuno)therapy in patients with clinically node-positive breast cancer. Clin Breast Cancer. 2014;14(5):315–22.
Choi HJ, Ryu JM, Kim I, et al. Nomogram for accurate prediction of breast and axillary pathologic response after neoadjuvant chemotherapy in node positive patients with breast cancer. Ann Surg Treat Res. 2019;96(4):169–76.
Kim JY, Park HS, Kim S, Ryu J, Park S, Kim SI. Prognostic nomogram for prediction of axillary pathologic complete response after neoadjuvant chemotherapy in cytologically proven node-positive breast cancer. Medicine (Baltimore). 2015;94(43):e1720.
Murphy BL, Day CN, Hoskin TL, Habermann EB, Boughey JC. Neoadjuvant chemotherapy use in breast cancer is greatest in excellent responders: triple-negative and HER2 + subtypes. Ann Surg Oncol. 2018;25(8):2241–8.
King TA, Morrow M. Surgical issues in patients with breast cancer receiving neoadjuvant chemotherapy. Nat Rev Clin Oncol. 2015;12(6):335–43.
Untch M, Konecny GE, Paepke S, von Minckwitz G. Current and future role of neoadjuvant therapy for breast cancer. Breast. 2014;23(5):526–37.
Murphy BL, Hoskin TL, Heins CDN, Habermann EB, Boughey JC. Preoperative prediction of node-negative disease after neoadjuvant chemotherapy in patients presenting with node-negative or node-positive breast cancer. Ann Surg Oncol. 2017;24(9):2518–25.
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310(14):1455–61.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–18.
Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol. 2015;33(3):258–64.
Steyerberg EW, Harrell FE Jr. Prediction models need appropriate internal, internal–external, and external validation. J Clin Epidemiol. 2016;69:245–47.
Kuerer HM, Sahin AA, Hunt KK, et al. Incidence and impact of documented eradication of breast cancer axillary lymph node metastases before surgery in patients treated with neoadjuvant chemotherapy. Ann Surg. 1999;230(1):72–8.
Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23(16):3676–85.
Dominici LS, Negron Gonzalez VM, Buzdar AU, et al. Cytologically proven axillary lymph node metastases are eradicated in patients receiving preoperative chemotherapy with concurrent trastuzumab for HER2-positive breast cancer. Cancer. 2010;116(12):2884–89.
Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005;23:9304–11.
Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) prospective multicenter clinical trial. Ann Surg. 2014;260(4):608–14 (discussion 614–6).
Donker M, Straver ME, Wesseling J, et al. Marking axillary lymph nodes with radioactive iodine seeds for axillary staging after neoadjuvant systemic treatment in breast cancer patients: the MARI procedure. Ann Surg. 2015;261(2):378–82.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
John Davis Jr, Tanya L. Hoskin, Courtney N. Day, Mark Wickre, Mara A. Piltin, Abigail S. Caudle, and Judy C. Boughey have no conflicts of interest or sources of funding to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Davis, J., Hoskin, T.L., Day, C.N. et al. Performance and Clinical Utility of Models Predicting Eradication of Nodal Disease in Patients with Clinically Node-Positive Breast Cancer Treated with Neoadjuvant Chemotherapy by Tumor Biology. Ann Surg Oncol 27, 4678–4686 (2020). https://doi.org/10.1245/s10434-020-08885-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08885-w