Abstract
Background
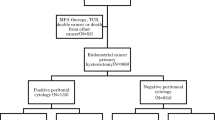
The prognosis for patients with endometrial cancer (EC) peritoneal carcinomatosis (PC) recurrence has received little study. This study aimed to determine specific risk factors and prognosis of EC with PC recurrence (PCR) versus no PC recurrence (NPCR).
Methods
Data of all patients with EC who received primary surgical treatment between January 2000 and February 2017 were abstracted from the French FRANCOGYN Research Group database. Clinical and pathologic variables were compared between the two groups (PCR vs. NPCR). Multivariate analysis was performed to define prognostic factors for peritoneal recurrence. Overall survivals (OS) of patients after recurrence were compared using the Kaplan–Meier method.
Results
The study analyzed 1466 patients, and 257 of these patients (17.5%) had recurrence. At presentation, 63 of these patients had PC. International Federation of Gynecology and Obstetrics (FIGO) stages 3 and 4 disease were significantly associated with PCR versus NPCR (odds ratio 2.24; 95% confidence interval 1.23–4.07; p = 0.008). The death rate for the patients with PC was 47.6%, with a median survival of 12 months after diagnosis of recurrence. According to the histologic subtype, OS was 29 months (Q1–Q3, 13–NA) for endometrioid carcinomas, 7.5 months (Q1–Q3, 4–15) for serous carcinomas, and 10 months (Q1–Q3, 5–15) for clear cell carcinomas. Chemotherapy for treatment of PCR was associated with improved OS after recurrence (OSAR; p = 0.0025).
Conclusion
An initial advanced stage of EC is a risk factor for PCR. For women with PCR, a diagnosis of type 1 EC recurrence more than 12 months after the initial treatment and management of PCR with chemotherapy is associated with improved OSAR. Prospective studies are needed to determine the precise optimal management required in this clinical situation and to assess the relevance of biomarkers to predict the risk of PCR for EC patients.




Similar content being viewed by others
References
Ferlay J, Parkin DM, Steliarova-Foucher E. Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer. 2010;46:765–81.
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JWW, Comber H, et al. Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer. 2013;49:1374–403.
Stewart BW, Wild CP. World Cancer Report 2014. 2014 [cité 11 sept 2018]. Disponible sur: http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2014.
Binder-Foucard F, Bossard N, Delafosse P, Belot A, Woronoff A-S, Remontet L. Cancer incidence and mortality in France over the 1980–2012 period: solid tumors. Rev d’Épidémiol Santé Publique. 2014;62:95–108.
Fung-Kee-Fung M, Dodge J, Elit L, Lukka H, Chambers A, Oliver T. Follow-up after primary therapy for endometrial cancer: a systematic review. Gynecol Oncol. 2006;101:520–9.
Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO–ESGO–ESTRO consensus conference on endometrial cancer: diagnosis, treatment, and follow-up. Radiother Oncol. 2015;117:559–81.
Querleu D, Darai E, Lecuru F, Rafii A, Chereau E, Collinet P, et al. Prise en charge primaire des cancers de l’endomètre: recommandations SFOG-CNGOF. Gynécol Obstét Fertilité Sénologie. 2017;45:715–25.
Colombo N, Creutzberg C, Amant F, Bosse T, González-Martín A, Ledermann J, et al. ESMO–ESGO–ESTRO Consensus conference on endometrial cancer: diagnosis, treatment, and follow-up. Int J Gynecol Cancer. 2016;26:2–30.
Bendifallah S, Canlorbe G, Raimond E, Bazire L, Huguet F, Graesslin O, et al. An external validation study of nomograms designed to predict isolated loco-regional and distant endometrial cancer recurrences: how applicable are they? Br J Cancer. 2013;109:1498–503.
Bendifallah S, Canlorbe G, Raimond E, Hudry D, Coutant C, Graesslin O, et al. External validation of nomograms designed to predict lymphatic dissemination in patients with early-stage endometrioid endometrial cancer: a multicenter study. Am J Obstet Gynecol. 2015;212:56.e1–e7.
Koskas M, Fournier M, Luton D, Darai E, Rouzier R. Survival impact of lymphadenectomy stratified by nodal metastatic probability in endometrial cancer. Ann Surg Oncol. 2014;21:2376–82.
Institut National du Cancer. Cancer de l’endometre, rapport integral. 2010. Disponible sur: http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Cancer-de-l-endometre-rapport-integral.
Bendifallah S, Ouldamer L, Lavoue V, Canlorbe G, Raimond E, Coutant C, et al. Patterns of recurrence and outcomes in surgically treated women with endometrial cancer according to ESMO–ESGO–ESTRO consensus conference risk groups: results from the FRANCOGYN study group. Gynecol Oncol. 2017;144:107–12.
Ahmed A, Zamba G, DeGeest K, Lynch CF. The impact of surgery on survival of elderly women with endometrial cancer in the SEER program from 1992–2002. Gynecol Oncol. 2008;111:35–40.
Hamilton T, Lanuke K, Mack LA, Temple WJ. Long-term follow-up in the treatment of peritoneal carcinomatosis. Am J Surg. 2011;201:650–4.
Bricou A, Bendifallah S, Daix-Moreux M, Ouldamer L, Lavoue V, Benbara A, et al. A proposal for a classification for recurrent endometrial cancer: analysis of a French multicenter database from the FRANCOGYN study group. Int J Gynecol Cancer. 2018;28:1278–84.
Frati A, Ballester M, Dubernard G, Bats AS, Heitz D, Mathevet P, et al. Contribution of lymphoscintigraphy for sentinel lymph node biopsy in women with early-stage endometrial cancer: results of the SENTI-ENDO study. Ann Surg Oncol. 2015;22:1980–6.
Ballester M, Dubernard G, Lécuru F, Heitz D, Mathevet P, Marret H, et al. Detection rate and diagnostic accuracy of sentinel-node biopsy in early-stage endometrial cancer: a prospective multicentre study (SENTI-ENDO). Lancet Oncol. 2011;12:469–76.
Creasman W. Revised FIGO staging for carcinoma of the endometrium. Int J Gynaecol Obstet. 2009;105:109.
Albertini A-F, Devouassoux-Shisheboran M, Genestie C. Pathology of endometrioid carcinoma. Bull Cancer. 2012;99:7–12.
Soslow RA, Tornos C, Park KJ, Malpica A, Matias-Guiu X, Oliva E, et al. Endometrial carcinoma diagnosis: use of FIGO grading and genomic subcategories in clinical practice. Int J Gynecol Pathol. 2019;38:S64–74.
Briët JM, Hollema H, Reesink N, Aalders JG, Mourits MJE, ten Hoor KA, et al. Lymphvascular space involvement: an independent prognostic factor in endometrial cancer. Gynecol Oncol. 2005;96:799–804.
Institut National du Cancer. Les traitements du cancer de l’endometre. 2013. Disponible sur: http://www.e-cancer.fr/Expertises-et-publications/Catalogue-des-publications/Les-traitements-du-cancer-de-l-endometre.
Baekelandt MM, Castiglione M. ESMO Guidelines Working Group, Endometrial carcinoma: ESMO clinical recommendations for diagnosis, treatment and follow-up. Ann Oncol. 2008;19(Suppl 2):19–20.
Haute Autorité de Santé. Cancer de l’endometre. 2011. Disponible sur: https://www.has-sante.fr/portail/jcms/c_1021574/fr/ald-n-30-cancer-de-l-endometre.
Sartori E, Pasinetti B, Carrara L, Gambino A, Odicino F, Pecorelli S. Pattern of failure and value of follow-up procedures in endometrial and cervical cancer patients. Gynecol Oncol. 2007;107:S241–7.
Creutzberg CL, Wárlám-Rodenhuis CC, van de Steen-Banasik E, Beerman H, van Lent M. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. Lancet. 2000;355:8.
Adjuvant external beam radiotherapy in the treatment of endometrial cancer (MRC ASTEC and NCIC CTG EN.5 randomised trials): pooled trial results, systematic review, and meta-analysis. Lancet. 2009;373:137–46.
Kasamatsu T, Shiromizu K, Takahashi M, Matsumoto K, Shirai T. Analysis of initial failure site and spread pattern in endometrial carcinoma: a Japanese experience. Int J Gynecol Cancer. 1999;9:470–6.
Murphy KT, Rotmensch J, Yamada SD, Mundt AJ. Outcome and patterns of failure in pathologic stages I–IV clear-cell carcinoma of the endometrium: implications for adjuvant radiation therapy. Int J Radiat Oncol Biol Phys. 2003;55:1272–6.
Shimamoto K, Saito T, Okadome M, Shimokawa M. Prognostic significance of the treatment-free interval in patients with recurrent endometrial cancer. Eur J Obstet Gynecol Reprod Biol. 2014;175:92–6.
Coleman RL, Brady MF, Herzog TJ, Sabbatini P, Armstrong DK, Walker JL, et al. Bevacizumab and paclitaxel–carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:779–91.
Aghajanian C, Goff B, Nycum LR, Wang YV, Husain A, Blank SV. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol Oncol. 2015;139:10–6.
Parmar MKB, Ledermann JA, Colombo N, du Bois A, Delaloye J-F, Kristensen GB, et al. Paclitaxel plus platinum-based chemotherapy versus conventional platinum-based chemotherapy in women with relapsed ovarian cancer: the ICON4/AGO-OVAR-2.2 trial. Lancet. 2003;361:2099–106.
Arend RC, Jones BA, Martinez A, Goodfellow P. Endometrial cancer: molecular markers and management of advanced stage disease. Gynecol Oncol. 2018;150:569–80.
Sagae S, Susumu N, Viswanathan AN, Aoki D, Backes FJ, Provencher DM, et al. Gynecologic Cancer InterGroup (GCIG) consensus review for uterine serous carcinoma. Int J Gynecol Cancer. 2014;24:S83–9.
Mhawech-Fauceglia P, Herrmann RF, Kesterson J, Izevbaye I, Lele S, Odunsi K. Prognostic factors in stages II/III/IV and stages III/IV endometrioid and serous adenocarcinoma of the endometrium. EJSO Eur J Surg Oncol. 2010;36:1195–201.
Getz G, Gabriel SB, Cibulskis K, Lander E, Sivachenko A, Sougnez C, et al. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497:67–73.
Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387:1094–108.
Phelippeau J, Rouzier R, Koskas M. Adherence to guidelines during follow-up of endometrial cancer: analysis of French Health Insurance Database. Anticancer Res. 2018;38:2977–82.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gaudet Chardonnet, A., Azaïs, H., Ballester, M. et al. Prognostic Value and Risk Factors of Peritoneal Carcinomatosis Recurrence for Patients with Endometrial Cancer: A Multicenter Study from the FRANCOGYN Group. Ann Surg Oncol 28, 212–221 (2021). https://doi.org/10.1245/s10434-020-08812-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08812-z