Abstract
Background
RAD18 plays an important role in DNA damage repair by inducing monoubiquitinated PCNA (mUB-PCNA) in both cancer and normal tissues. Previous studies have not determined the significance of RAD18 expression in clinical gastric cancer (GC) samples. Thus, this study aimed to clarify the expression and functional significance of RAD18 in GC.
Methods
Overall, 96 resected GC samples were subjected to an immunohistochemical analysis of RAD18. GC cell lines were also subjected to functional RNA interference analyses of RAD18.
Results
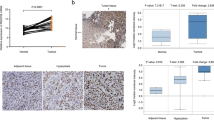
RAD18 expression was predominantly nuclear and was observed at higher levels in GC tissues than in normal tissues. In GC tissues, strong RAD18 expression was associated with progression of lymph node metastasis (p = 0.0001), lymphatic invasion (p = 0.0255), venous invasion (p < 0.0001), recurrence (p = 0.028), and disease stage (p = 0.0253). Moreover, GC patients with high tumor RAD18 expression had shorter overall survival (p = 0.0061) and recurrence-free survival durations (p = 0.035) than those with low tumor RAD18 expression. RAD18 knockdown inhibited GC proliferation and invasiveness and increased chemosensitivity by suppressing mUB-PCNA.
Conclusions
RAD18 expression may be a useful marker of progression and poor prognosis of GC. Moreover, therapeutic strategies that target RAD18 might be a novel chemosensitizer to eradicate the refractory GC.




Similar content being viewed by others
References
Carlomagno N, Incollingo P, Tammaro V, et al. Diagnostic, predictive, prognostic, and therapeutic molecular biomarkers in third millennium: a breakthrough in gastric cancer. Biomed Res Int. 2017;2017:7869802.
Cao J, Qi F, Liu T. Adjuvant chemotherapy after curative resection for gastric cancer: a meta-analysis. Scand J Gastroenterol. 2014;49(6):690–704.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
Varanasi L, Do PM, Goluszko E, Martinez LA. Rad18 is a transcriptional target of E2F3. Cell Cycle. 2012;11(6):1131–41.
Hibbert RG, Huang A, Boelens R, Sixma TK. E3 ligase Rad18 promotes monoubiquitination rather than ubiquitin chain formation by E2 enzyme Rad6. Proc Natl Acad Sci USA. 2011;108(14):5590–5.
Zou S, Yang J, Guo J, et al. RAD18 promotes the migration and invasion of esophageal squamous cell cancer via the JNK-MMPs pathway. Cancer Lett. 2018;417:65–74.
Suzuki M, Yokobori T, Gombodorj N, et al. High stromal transforming growth factor beta-induced expression is a novel marker of progression and poor prognosis in gastric cancer. J Surg Oncol. 2018;118(6):966–74.
Zhang W, Qin Z, Zhang X, Xiao W. Roles of sequential ubiquitination of PCNA in DNA-damage tolerance. FEBS Lett. 2011;585(18):2786–94.
Hedglin M, Aitha M, Pedley A, Benkovic SJ. Replication protein A dynamically regulates monoubiquitination of proliferating cell nuclear antigen. J Biol Chem. 2019;294(13):5157–68.
Garg P, Burgers PM. Ubiquitinated proliferating cell nuclear antigen activates translesion DNA polymerases eta and REV1. Proc Natl Acad Sci USA. 2005;102(51):18361–6.
Nakamura T, Ishikawa S, Koga Y, et al. Mutation analysis of Rad18 in human cancer cell lines and non small cell lung cancer tissues. J Exp Clin Cancer Res. 2009;28:106.
Durando M, Tateishi S, Vaziri C. A non-catalytic role of DNA polymerase eta in recruiting Rad18 and promoting PCNA monoubiquitination at stalled replication forks. Nucleic Acids Res. 2013;41(5):3079–93.
Shiomi N, Mori M, Tsuji H, et al. Human RAD18 is involved in S phase-specific single-strand break repair without PCNA monoubiquitination. Nucleic Acids Res. 2007;35(2):e9.
Watanabe K, Tateishi S, Kawasuji M, Tsurimoto T, Inoue H, Yamaizumi M. Rad18 guides poleta to replication stalling sites through physical interaction and PCNA monoubiquitination. EMBO J. 2004;23(19):3886–96.
Liu T, Chen H, Kim H, Huen MS, Chen J, Huang J. RAD18-BRCTx interaction is required for efficient repair of UV-induced DNA damage. DNA Repair (Amst). 2012;11(2):131–8.
Yan X, Chen J, Meng Y, et al. RAD18 may function as a predictor of response to preoperative concurrent chemoradiotherapy in patients with locally advanced rectal cancer through caspase-9-caspase-3-dependent apoptotic pathway. Cancer Med. 2019;8(6):3094–104.
Lou P, Zou S, Shang Z, et al. RAD18 contributes to the migration and invasion of human cervical cancer cells via the interleukin1beta pathway. Mol Med Rep. 2019;20(4):3415–23.
Wong RP, Aguissa-Toure AH, Wani AA, et al. Elevated expression of Rad18 regulates melanoma cell proliferation. Pigment Cell Melanoma Res. 2012;25(2):213–8.
Acknowledgement
The authors express deep gratitude to the Rotary Club Foundation for financial assistance during the course of the study. This study was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (JSPS; Grant Numbers 17K19893, 18K07665, and 18H02877). This work was also supported in part by the Research Grant of the Princess Takamatsu Cancer Research Fund, Suzuken Memorial Foundation, and Pancreas Research Foundation of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study conformed to the tenets of the Declaration of Helsinki and was approved by the Institutional Review Board for Clinical Research at Gunma University Hospital (Maebashi, Gunma, Japan; approval number HS2019-043). Patient consent was obtained using the opt-out method.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1
Overall survival curve of 631 GC samples from the online microarray database (www.kmplot.com) according to RAD18 mRNA expression. GC patients with high levels of RAD18 mRNA expression (n = 369) exhibited worse survival outcomes compared with GC patients with low levels of RAD18 mRNA expression (n = 262) (p = 0.0098). (TIFF 28950 kb)
Supplementary Figure 2
(a) Kaplan–Meier analysis of overall survival in our cohort of GC patients (n = 34). The analyses were based on RAD18 expression (p = 0.845). (B) Kaplan–Meier analyses of recurrence-free survival (n = 34) according to RAD18 expression (p = 0.884). The overall survival and recurrence-free survival rates were evaluated insignificant value in GC patients. (TIFF 28950 kb)
Rights and permissions
About this article
Cite this article
Baatar, S., Bai, T., Yokobori, T. et al. High RAD18 Expression is Associated with Disease Progression and Poor Prognosis in Patients with Gastric Cancer. Ann Surg Oncol 27, 4360–4368 (2020). https://doi.org/10.1245/s10434-020-08518-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-020-08518-2