Abstract
Background
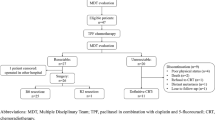
The optimal treatment strategy for patients with borderline resectable (BR) esophageal squamous cell carcinoma (ESCC), in which tumors grow very close to the adjacent vital organs, remains unclear. This study evaluated the efficacy of neoadjuvant chemoradiotherapy (NACRT) with cisplatin plus fluorouracil (CF) and irradiation (40 Gy) for these patients.
Methods
The study cohort included 50 patients with BR-ESCC who received NACRT as the initial treatment and were allocated to one of two groups: patients who achieved curative resection (R0 group) or those who did not (Non-R0 group). The overall survival (OS), relapse-free survival (RFS), and pre-therapeutic predictive factors for Non-R0 were evaluated.
Results
Among the 50 patients, 22 (44%) achieved curative resection clinically. The median OS was significantly better in the R0 group than in the Non-R0 group (2.4 vs 0.8 years; hazard ratio [HR], 0.29; 95% confidence interval [CI], 0.12–0.67; p < 0.01). The independent predictive factors before NACRT for Non-R0 were higher serum SCC antigen level (p < 0.01) and clinical nodal involvement (p = 0.02). In addition, OS was significantly worse for the patients with higher levels of serum SCC antigen than for those with lower levels (p < 0.01).
Conclusions
Curative resection was achieved for about 40% of the patients who received NACRT for BR-ESCC. Therefore, NACRT could be a useful neoadjuvant treatment option for BR-ESCC. However, a higher serum SCC antigen level before NACRT is predictive of treatment failure and poor survival.




Similar content being viewed by others
References
GLOBOCAN database. http://gco.iarc.fr/. Accessed 8 July 2019.
Surveillance, epidemiology, and end results program. https://seer.cancer.gov/statfacts/html/esoph.html. Accessed 8 July 2019.
Sasaki Y, Tamura M, Koyama R, et al. Genomic characterization of esophageal squamous cell carcinoma: insights from next-generation sequencing. World J Gastroenterol. 2016;22:2284–93.
Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19:68–74.
Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017, edited by the Japan Esophageal Society: Part 1. Esophagus. 2019;16:1–24.
Ohtsu A, Boku N, Muro K, et al. Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17:2915–21.
Kitagawa Y, Uno T, Oyama T, et al. Esophageal cancer practice guidelines 2017, edited by the Japan Esophageal Society: part 2. Esophagus. 2019;16:25–43.
Ishida K, Ando N, Yamamoto S, et al. Phase II study of cisplatin and 5-fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG)/Japan Clinical Oncology Group Trial (JCOG9516). Jpn J Clin Oncol. 2004;34:615–9.
Shinoda M, Ando N, Kato K, et al. Randomized study of low-dose versus standard-dose chemoradiotherapy for unresectable esophageal squamous cell carcinoma (JCOG0303). Cancer Sci. 2015;106:407–12.
Burmeister BH, Smithers BM, Gebski V, et al. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomized controlled phase III trial. Lancet Oncol. 2005;6:659–68.
Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol. 2008;26:1086–92.
Mariette C, Dahan L, Mornex F, et al. Surgery alone versus chemoradiotherapy followed by surgery for stage I and II esophageal cancer: final analysis of randomized controlled phase III trial FFCD 9901. J Clin Oncol. 2014;32:2416–22.
van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med. 2012;366:2074–84.
Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol. 2011;12:681–92.
Picus D, Balfe DM, Koehler RE, et al. Computed tomography in the staging of esophageal carcinoma. Radiology. 1983;146:433–8.
Noguchi T, Moriyama H, Wada S, et al. Resection surgery with neoadjuvant chemoradiotherapy improves outcomes of patients with T4 esophageal carcinoma. Dis Esophagus. 2003;16:94–8.
Fujita H, Sueyoshi S, Tanaka T, et al. Prospective non-randomized trial comparing esophagectomy followed by chemoradiotherapy versus chemoradiotherapy followed by esophagectomy for T4 esophageal cancers. J Surg Oncol. 2005;90:209–19.
Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
Forrest LM, McMillan DC, McArdle CS, Angerson WJ, Dunlop DJ. Evaluation of cumulative prognostic scores based on the systemic inflammatory response in patients with inoperable non-small cell lung cancer. Br J Cancer. 2003;89:1028–30.
Yodying H, Matsuda A, Miyashita M, et al. Prognostic significance of neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio in oncologic outcomes of esophageal cancer: a systematic review and meta-analysis. Ann Surg Oncol. 2016;23:646–54.
Common Terminology Criteria for Adverse Events (CTCAE) v 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Accessed 18 August 2019.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13.
Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–5.
Yokota T, Hatooka S, Ura T, et al. Docetaxel plus 5-fluorouracil and cisplatin (DCF) induction chemotherapy for locally advanced borderline-resectable T4 esophageal cancer. Anticancer Res. 2011;31:3535–41.
Watanabe M, Baba Y, Yoshida N, et al. Outcomes of preoperative chemotherapy with docetaxel, cisplatin, and 5-fluorouracil followed by esophagectomy in patients with resectable node-positive esophageal cancer. Ann Surg Oncol. 2014;21:2838–44.
Takeuchi M, Kawakubo H, Mayanagi S, et al. The benefits of docetaxel plus cisplatin and 5-fluorouracil induction therapy in conversion to curative treatment for locally advanced esophageal squamous cell carcinoma. World J Surg. 2019;43:2006–15.
Yokota T, Kato K, Hamamoto Y, et al. Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer. 2016;115:1328–34.
Terada M, Hara H, Daiko H, et al. Phase III study of tri-modality combination therapy with induction docetaxel plus cisplatin and 5-fluorouracil versus definitive chemoradiotherapy for locally advanced unresectable squamous cell carcinoma of the thoracic esophagus (JCOG1510: TRIANgLE). Jpn J Clin Oncol. (2019). https://doi.org/10.1093/jjco/hyz112
Torre GC. SCC antigen in malignant and nonmalignant squamous lesions. Tumour Biol. 1998;19:517–26.
Shimada H, Nabeya Y, Okazumi S, et al. Prediction of survival with squamous cell carcinoma antigen in patients with resectable esophageal squamous cell carcinoma. Surgery. 2003;133:486–94.
Okamura A, Watanabe M, Mine S, et al. Failure of neoadjuvant chemotherapy for resectable esophageal squamous cell carcinoma. Dis Esophagus. 2017;30:1–8.
Hamamoto Y, Nojima M, Aoki Y, et al. Inter-evaluator heterogeneity of clinical diagnosis for locally advanced esophageal squamous cell carcinoma. Esophagus. 2017;14:324–32.
Yokota T, Yasuda T, Kato H. et al. Concordance of clinical diagnosis of T classification among physicians for locally advanced unresectable thoracic esophageal cancer. Int J Clin Oncol. 2018;23:73–80.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
All authors declare no conflicts of interests for this article. Conflicts of interests outside this work are as follows; Kensei Yamaguchi played Consulting or Advisory Role for Bristol-Myers Squibb Japan and Daiichi Sankyo, and received Speakers’ Bureau from Chugai Pharma, Merck Serono, Bristol-Myers Squibb Japan, Takeda, Taiho Pharmaceutical, Lilly, Ono Pharmaceutical, Sanofi and Daiichi Sankyo. Kensei Yamaguchi’s institution (Department of Gastroenterological Medicine, The Cancer Institute Hospital of the Japanese Foundation for Cancer Research) received research funding from MSD Oncology, Ono Pharmaceutical, Dainippon Sumitomo Pharma, Taiho Pharmaceutical, Daiichi Sankyo, Lilly, Gilead Sciences, Yakult Honsha, Chugai Pharma, Boehringer Ingelheim and Eisai.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Suzuki, T., Okamura, A., Watanabe, M. et al. Neoadjuvant Chemoradiotherapy with Cisplatin Plus Fluorouracil for Borderline Resectable Esophageal Squamous Cell Carcinoma. Ann Surg Oncol 27, 1510–1517 (2020). https://doi.org/10.1245/s10434-019-08124-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-08124-x