Abstract
Background
The American Joint Committee on Cancer (AJCC) breast cancer pathological prognostic stage, which incorporates biologic factors, was developed using data from patients undergoing upfront surgery, and its application in patients receiving neoadjuvant chemotherapy (NAC) is unknown. We previously developed the Neo-Bioscore, incorporating clinical and pathological TNM categories with biologic factors, to improve the prognostic stratification of NAC patients.
Objective
This study was undertaken to evaluate the use of available staging models incorporating biologic factors in NAC patients.
Methods
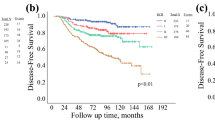
Patients treated with NAC between 2005 and 2012 at MD Anderson (n = 2363) were staged using the Neo-Bioscore and the AJCC 8th edition: (1) clinical anatomic stage; (2) pathological anatomic stage; (3) clinical prognostic stage; and (4) pathological prognostic stage. Five-year disease-specific survival (DSS) and overall survival (OS) rates, along with Harrell’s concordance index (C-index), were estimated. A National Cancer Database (NCDB) cohort (n = 12,887) treated with NAC between 2010 and 2013 was used for validation.
Results
In the MD Anderson cohort, staging systems incorporating biologic factors better predicted DSS (bias-corrected C-index: pathological prognostic stage = 0.8026; Neo-Bioscore = 0.7483) and OS (bias-corrected C-index: pathological prognostic stage = 0.7780; Neo-Bioscore = 0.7260) than those using anatomic factors only. Similar results were seen in the NCDB cohort. In pairwise comparisons, the pathological prognostic stage was significantly better (p < 0.0001) than other staging systems in all comparisons except for OS in the NCDB cohort, where it was not significantly different than the Neo-Bioscore (p = 0.2).
Conclusion
Biologic factors are important for determining prognosis in patients receiving NAC. These data indicate that the 8th edition AJCC pathological prognostic stage is applicable in these patients.


Similar content being viewed by others
References
Hortobagyi GN, Connolly JL, D’Orsi CJ, et al. AJCC Cancer Staging Manual, 8th Edition Updates and Corrections. AJCC Breast Cancer Staging System, 2017.
Weiss A, Chavez-MacGregor M, Lichtensztajn DY, et al. Validation study of the American Joint Committee on Cancer eighth edition prognostic stage compared with the anatomic stage in breast cancer. JAMA Oncol. 2018;4(2):203–9.
Jeruss JS, Mittendorf EA, Tucker SL, et al. Combined use of clinical and pathologic staging variables to define outcomes for breast cancer patients treated with neoadjuvant therapy. J Clin Oncol. 2008;26(2):246–52.
Mittendorf EA, Jeruss JS, Tucker SL, et al. Validation of a novel staging system for disease-specific survival in patients with breast cancer treated with neoadjuvant chemotherapy. J Clin Oncol. 2011;29(15):1956–62.
Mittendorf EA, Vila J, Tucker SL, et al. The Neo-Bioscore update for staging breast cancer treated with neoadjuvant chemotherapy: Incorporation of prognostic biologic factors into staging after treatment. JAMA Oncol. 2016;2(7):929–36.
Bergquist JR, Murphy BL, Storlie CB, Habermann EB, Boughey JC. Incorporation of treatment response, tumor grade and receptor status improves staging quality in breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol. 2017;24(12):3510–7.
Hammond ME, Hayes DF, Wolff AC, Mangu PB, Temin S. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6(4):195–7.
Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013.
Kaplan EL, Meier P. Nonparametric-estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–81.
Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163–70.
Cox DR. Regression models and life-tables. J R Stat Soc Ser B Stat Methodol. 1972;34(2):187–220.
Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143(1):29–36.
Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer; 2001.
Harrell FE Jr, Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the yield of medical tests. JAMA. 1982;247(18):2543–6.
Kang L, Chen WJ, Petrick NA, Gallas BD. Comparing two correlated C indices with right-censored survival outcome: a one-shot nonparametric approach. Stat Med. 2015;34(4):685–703.
Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. Ann Surg. 2014;260(4):608–14 (discussion 14–6).
Symmans WF, Wei C, Gould R, et al. Long-term prognostic risk after neoadjuvant chemotherapy associated with residual cancer burden and breast cancer subtype. J Clin Oncol. 2017;35(10):1049–60.
Symmans WF, Peintinger F, Hatzis C, et al. Measurement of residual breast cancer burden to predict survival after neoadjuvant chemotherapy. J Clin Oncol. 2007;25(28):4414–22.
von Minckwitz G, Untch M, Blohmer JU. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012;30(15):1796–804.
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–72.
Funding
This study was supported in part by the MD Anderson Cancer Center National Cancer Institute Cancer Center support Grant P30 CA016672.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Kelly K. Hunt reports compensated participation in medical advisory boards for Armada Health and Merck; Mariana Chavez-MacGregor reports having served as a consultant for Abbott, Eisai, Pfizer and Roche; Tari A. King reports speaker fees from Genomic Health; and Elizabeth A. Mittendorf reports compensated participation in scientific advisory boards for Amgen, Celgene, Astra-Zeneca, Genentech, Genomic Health, Merck, Sellas Life Sciences, and Tapimmune. The other authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yi, M., Lin, H., Bedrosian, I. et al. Staging for Breast Cancer Patients Receiving Neoadjuvant Chemotherapy: Utility of Incorporating Biologic Factors. Ann Surg Oncol 27, 359–366 (2020). https://doi.org/10.1245/s10434-019-07983-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07983-8