Abstract
Background
Dysphagia is a major symptom of esophageal cancer (EC) that significantly affects patient quality of life; however, little is known regarding its clinical impact on the treatment course in patients with EC.
Methods
This retrospective study included 434 consecutive patients with EC who received docetaxel, cisplatin, and 5-fluorouracil (DCF) chemotherapy as an initial treatment. We evaluated the relationships between the dysphagia score at diagnosis and clinicopathological factors, including DCF therapy-related adverse events, tumor response, and survival.
Results
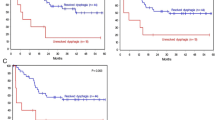
The dysphagia scores were 0 in 208 patients (47.9%), 1 in 82 patients (18.9%), 2 in 52 patients (12.0%), 3 in 59 patients (13.6%), and 4 in 33 patients (7.6%). High (≥ 3) dysphagia scores were significantly associated with high incidences of grade 3/4 febrile neutropenia (FN) (79.3 vs. 35.7%, P < 0.001) and diarrhea (63.0 vs. 28.1%, P < 0.001) compared with low (≤ 2) scores. Logistic regression analysis further identified the dysphagia scores as an independent predictor of both FN and severe diarrhea during DCF chemotherapy. Furthermore, compared with low scores, high dysphagia scores were associated with a worse clinical response to chemotherapy (response rate 65.2 vs. 78.7%, P = 0.008) and worse 5-year overall survival (35.4 vs. 56.4%, P = 0.001).
Conclusions
The dysphagia score at diagnosis was an independent predictor of FN and severe diarrhea. Furthermore, this score might be useful in predicting chemotherapy response and long-term survival in patients treated with DCF.



Similar content being viewed by others
References
Ando N, Kato H, Igaki H, et al. A randomized trial comparing postoperative adjuvant chemotherapy with cisplatin and 5-fluorouracil versus preoperative chemotherapy for localized advanced squamous cell carcinoma of the thoracic esophagus (JCOG9907). Ann Surg Oncol. 2012;19(1):68–74.
Allum WH, Stenning SP, Bancewicz J, et al. Long-term results of a randomized trial of surgery with or without preoperative chemotherapy in esophageal cancer. J Clin Oncol. 2009;27:5062–7.
Kelsen DP, Winter KA, Gunderson LL, et al. Radiation Therapy Oncology Group; USA Intergroup. Long-term results of RTOG trial 8911 (USA Intergroup 113): a random assignment trial comparison of chemotherapy followed by surgery compared with surgery alone for esophageal cancer. J Clin Oncol. 2007;25:3719–25.
Yamasaki M, Miyata H, Miyazaki Y, et al. Perioperative therapy for esophageal cancer. Gen Thorac Cardiovasc Surg. 2014;62:531–40.
Posner MR, Hershock DM, Blajman CR, et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med. 2007;357:1705–15.
Vermorken JB, Remenar E, van Herpen C, et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med. 2007;357:1695–704.
Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997.
Yamasaki M, Miyata H, Tanaka K, et al. Multicenter phase I/II study of docetaxel, cisplatin and fluorouracil combination chemotherapy in patients with advanced or recurrent squamous cell carcinoma of the esophagus. Oncology. 2011;80:307–13.
Yamasaki M, Yasuda T, Yano M, et al. Multicenter randomized phase II study of cisplatin and fluorouracil plus docetaxel (DCF) compared with cisplatin and fluorouracil plus Adriamycin (ACF) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (OGSG1003). Ann Oncol. 2017;28:116–20.
Hara H, Tahara M, Daiko H, et al. Phase II feasibility study of preoperative chemotherapy with docetaxel, cisplatin, and fluorouracil for esophageal squamous cell carcinoma. Cancer Sci. 2013;104:1455–60.
Yokota T, Kato K, Hamamoto Y, et al. Phase II study of chemoselection with docetaxel plus cisplatin and 5-fluorouracil induction chemotherapy and subsequent conversion surgery for locally advanced unresectable oesophageal cancer. Br J Cancer. 2016;115:1328–34.
Shimanuki M, Imanishi Y, Sato Y, et al. Pretreatment monocyte counts and neutrophil counts predict the risk for febrile neutropenia in patients undergoing TPF chemotherapy for head and neck squamous cell carcinoma. Oncotarget. 2018;9:18970–84.
Takenaka Y, Cho H, Yamamoto M, et al. Incidence and predictors of febrile neutropenia during chemotherapy in patients with head and neck cancer. Support Care Cancer. 2013;21:2861–8.
Mellow MH, Pinkas H. Endoscopic laser therapy for malignancies affecting the esophagus and gastroesophageal junction: analysis of technical and functional efficacy. Arch Intern Med. 1985;145:1443–6.
Knyrim K, Wagner HJ, Bethge N, et al. A controlled trial of an expansile metal stent for palliation of esophageal obstruction due to inoperable cancer. N Engl J Med. 1993;329:1302–7.
Watkinson AF, Ellul J, Entwisle K, et al. Esophageal carcinoma: initial results of palliative treatment with covered self-expanding endoprostheses. Radiology. 1995;195:821–7.
Maunoury V, Brunetaud JM, Cochelard D, et al. Endoscopic palliation for inoperable malignant dysphagia: long term follow up. Gut. 1992;33:1602–7.
Oken M, Creech R, Tormey D, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55.
Yang CJ, Roh JL, Choi KH, et al. Pretreatment dysphagia inventory and videofluorographic swallowing study as prognostic indicators of early survival outcomes in head and neck cancer. Cancer. 2015;121:1588–98.
Brierley J, Gospodarowicz M, Wittekind C. TNM classification of malignant tumors. 8th ed. Oxford: Wiley-Blackwell; 2017.
Japan Esophageal Society. Japanese classification of esophageal cancer, 11th Edn: part II and III. Esophagus. 2017;14:37–65.
Makino T, Yamasaki M, Tanaka K, et al. Metabolic tumor volume change predicts long-term survival and histological response to preoperative chemotherapy in locally advanced esophageal cancer. Ann Surg. 2018. https://doi.org/10.1097/sla.0000000000002808.
Hashimoto T, Makino T, Yamasaki Y, et al. The pattern of residual tumor after neoadjuvant chemotherapy for locally advanced esophageal cancer and its clinical significance. Ann Surg. 2019. https://doi.org/10.1097/sla.0000000000003129.
Makino T, Yamasaki M, Tanaka K, et al. Importance of positron emission tomography for assessing the response of primary and metastatic lesions to induction treatments in T4 esophageal cancer. Surgery. 2017;162:836–45.
Makino T, Yamasaki M, Tanaka K, et al. Utility of initial induction chemotherapy with 5-fluorouracil, cisplatin, and docetaxel (DCF) for T4 esophageal cancer: a propensity score-matched analysis. Dis Esophagus. 2018;31(4):130.
Makino T, Miyata H, Yamasaki M, et al. Utility of response evaluation to neo-adjuvant chemotherapy by 18F-fluorodeoxyglucose-positron emission tomography in locally advanced esophageal squamous cell carcinoma. Surgery. 2010;148(5):908–18.
Makino T, Doki Y, Miyata H, et al. Use of (18)F-fluorodeoxyglucose-positron emission tomography to evaluate responses to neo-adjuvant chemotherapy for primary tumor and lymph node metastasis in esophageal squamous cell carcinoma. Surgery. 2008;144(5):793–802.
National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) version 5.0. Bethesda, MD: National Cancer Institute; 2017.
Arends J, Bachmann P, Baracos V, et al. ESPEN guidelines on nutrition in cancer patients. Clin Nutr. 2017;36:11–48.
Flowers CR, Seidenfeld J, Bow EJ, et al. Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;20:794–810.
Bistrian BR, Blackburn GL, Vitale J, et al. Prevalence of malnutrition in general medical patients. JAMA. 1976;235(15):1567–70.
EA Deitch. Bacterial translocation: the influence of dietary variables. Gut. 1994;35(1 Suppl):S23–7.
Sonis ST. The pathobiology of mucositis. Nat Rev Cancer. 2004;4:277–84.
van der Velden WJ, Herbers AH, Netea MG, et al. Mucosal barrier injury, fever and infection in neutropenic patients with cancer: introducing the paradigm febrile mucositis. Br J Haematol. 2014;167:441–52.
Miyata H, Yano M, Yasuda T, et al. Randomized study of clinical effect of enteral nutrition support during neoadjuvant chemotherapy on chemotherapy-related toxicity in patients with esophageal cancer. Clin Nutr. 2012;31:330–6.
Motoori M, Yano M, Miyata H, et al. Randomized study of the effect of synbiotics during neoadjuvant chemotherapy on adverse events in esophageal cancer patients. Clin Nutr. 2017;36:93–9.
Stephens MR, Lewis WG, White S, et al. Prognostic significance of alarm symptoms in patients with gastric cancer. Br J Surg. 2005;92:840–6.
Sánchez-Bueno F, Garcia-Marcilla JA, Perez-Flores D, et al. Prognostic factors in a series of 297 patients with gastric adenocarcinoma undergoing surgical resection. Br J Surg. 1998;85:255–60.
Deans DA, Tan BH, Wigmore SJ, et al. The influence of systemic inflammation, dietary intake and stage of disease on rate of weight loss in patients with gastro-oesophageal cancer. Br J Cancer. 2009;100:63–9.
Crawford J, Dale DC, Kuderer NM, et al. Risk and timing of neutropenic events in adult cancer patients receiving chemotherapy: the results of a prospective nationwide study of oncology practice. J Natl Compr Cancer Netw. 2008;6:109–18.
Andreyev HJ, Norman AR, Oates J, et al. Why do patients with weight loss have a worse outcome when undergoing chemotherapy for gastrointestinal malignancies? Eur J Cancer. 1998;34:503–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Takaomi Hagi, Tomoki Makino, Makoto Yamasaki, Koji Tanaka, Naohiro Nishida, Daisuke Sakai, Masaaki Motoori, Yutaka Kimura, Taroh Satoh, Masaki Mori, and Yuichiro Doki have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hagi, T., Makino, T., Yamasaki, M. et al. Dysphagia Score as a Predictor of Adverse Events Due to Triplet Chemotherapy and Oncological Outcomes in 434 Consecutive Patients with Esophageal Cancer. Ann Surg Oncol 26, 4754–4764 (2019). https://doi.org/10.1245/s10434-019-07744-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07744-7