Abstract
Background
Peritumoral lymphoid aggregates, termed Crohn’s-like lymphoid reaction (CLR), are markers of an antitumor immune response, which is an important predictor of patient outcome. In this study, we investigated the prognostic utility of CLR and its relationship with nutritional status in patients with gastric cancer (GC).
Methods
The study included 170 patients who underwent curative surgery for pathological stage (pStage) II/III GC. The maximum diameters of peritumoral and normal mucosal CLR aggregates were measured, and the median peritumoral diameter (0.57 mm) was used to stratify patients into two groups (large-CLR and small-CLR). The relationships between CLR size and preoperative nutritional status (body mass index, body composition status, Onodera’s prognostic nutritional index), tumor-infiltrating CD8+ T-lymphocyte count, and survival were evaluated.
Results
Peritumoral CLR aggregates were significantly larger than aggregates in the normal mucosa. Clinicopathological variables were not significantly different between the two patient groups; however, the large-CLR group had better cancer-specific survival (p = 0.018) and recurrence-free survival (p = 0.03) than the small-CLR group. Multivariate analysis revealed that CLR size was an independent prognostic factor for cancer-specific survival [hazard ratio (HR) 2.13, 95% confidence interval (CI) 1.3–3.56, p = 0.002] and recurrence-free survival (HR 1.96, 95% CI 1.22–3.19, p = 0.005). Nutritional status markers were significantly poorer for the small-CLR group than the large-CLR group. CD8+ T-cell tumor infiltration was positively correlated with CLR size but not with patient survival.
Conclusions
CLR size correlated with patient nutritional status and prognosis and may be helpful in identifying high-risk populations of pStage II/III GC patients.


Similar content being viewed by others
References
Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359–86.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: Cancer J Clin. 2015;65(2):87–108.
Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–20.
Suzuki Y, Oshima T, Yoshihara K, et al. Clinical significance of secreted protein, acidic and cysteine-rich gene expression in patients with stage II/III gastric cancer following curative resection and adjuvant chemotherapy with S-1. Oncol Lett. 2018;15(5):7335–43.
Kang YK, Boku N, Satoh T, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;390(10111):2461–71.
Duruisseaux M, Martinez-Cardus A, Calleja-Cervantes ME, et al. Epigenetic prediction of response to anti-PD-1 treatment in non-small-cell lung cancer: a multicentre, retrospective analysis. Lancet Respir Med. 2018;6(10):771–81.
Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366(26):2455–65.
Graham DM, Appelman HD. Crohn’s-like lymphoid reaction and colorectal carcinoma: a potential histologic prognosticator. Mod Pathol. 1990;3(3):332–5.
Kim JH, Kim KJ, Bae JM, et al. Comparative validation of assessment criteria for Crohn-like lymphoid reaction in colorectal carcinoma. J Clin Pathol. 2015;68(1):22–8.
Klintrup K, Makinen JM, Kauppila S, et al. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41(17):2645–54.
Ogino S, Nosho K, Irahara N, et al. Lymphocytic reaction to colorectal cancer is associated with longer survival, independent of lymph node count, microsatellite instability, and CpG island methylator phenotype. Clin Cancer Res. 2009;15(20):6412–20.
Rozek LS, Schmit SL, Greenson JK, et al. Tumor-infiltrating lymphocytes, Crohn’s-like lymphoid reaction, and survival from colorectal cancer. J Natl Cancer Inst. 2016. https://doi.org/10.1093/jnci/djw027.
Ueno H, Hashiguchi Y, Shimazaki H, et al. Objective criteria for crohn-like lymphoid reaction in colorectal cancer. Am J Clin Pathol. 2013;139(4):434–41.
Vayrynen JP, Sajanti SA, Klintrup K, et al. Characteristics and significance of colorectal cancer associated lymphoid reaction. Int J Cancer. 2014;134(9):2126–35.
Siewert JR, Bottcher K, Stein HJ, Roder JD. Relevant prognostic factors in gastric cancer: ten-year results of the German Gastric Cancer Study. Ann Surg. 1998;228(4):449–61.
Greenson JK, Huang SC, Herron C, et al. Pathologic predictors of microsatellite instability in colorectal cancer. Am J Surg Pathol. 2009;33(1):126–33.
Jenkins MA, Hayashi S, O’Shea AM, et al. Pathology features in Bethesda guidelines predict colorectal cancer microsatellite instability: a population-based study. Gastroenterology. 2007;133(1):48–56.
Raedle J, Trojan J, Brieger A, et al. Bethesda guidelines: relation to microsatellite instability and MLH1 promoter methylation in patients with colorectal cancer. Ann Intern Med. 2001;135(8 Pt 1):566–576.
Hirahara N, Tajima Y, Fujii Y, et al. Prognostic nutritional index as a predictor of survival in resectable gastric cancer patients with normal preoperative serum carcinoembryonic antigen levels: a propensity score matching analysis. BMC Cancer. 2018;18(1):285.
Kanda M, Mizuno A, Tanaka C, et al. Nutritional predictors for postoperative short-term and long-term outcomes of patients with gastric cancer. Medicine. 2016;95(24):e3781.
Wang HX, Wang CC, Yang W, Gao LL, Yu SQ. Prognostic value of preoperative prognostic nutritional index in stage III gastric cancer after curative resection: a retrospective cohort study. Asia Pac J Clin Nutr. 2018;27(3):540–5.
Prieto I, Montemuino S, Luna J, de Torres MV, Amaya E. The role of immunonutritional support in cancer treatment: current evidence. Clin Nutr. 2017;36(6):1457–64.
Shigemori T, Toiyama Y, Okugawa Y, et al. Soluble PD-L1 expression in circulation as a predictive marker for recurrence and prognosis in gastric cancer: direct comparison of the clinical burden between tissue and serum PD-L1 expression. Ann Surg Oncol. 2019;26(3):876–83.
Kono T, Sakamoto K, Shinden S, Ogawa K. Pre-therapeutic nutritional assessment for predicting severe adverse events in patients with head and neck cancer treated by radiotherapy. Clin Nutr. 2017;36(6):1681–5.
Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients [in Japanese]. Nihon Geka Gakkai zasshi. 1984;85(9):1001–5.
Okugawa Y, Toiyama Y, Yamamoto A, et al. Clinical impact of muscle quantity and quality in colorectal cancer patients: a propensity score matching analysis. J Parenter Enter Nutr. 2018;42(8):1322–33.
Okugawa Y, Toiyama Y, Yamamoto A, et al. Close relationship between immunological/inflammatory markers and myopenia and myosteatosis in patients with colorectal cancer: a propensity score matching analysis. J Parenter Enter Nutr. 2019;43(4):508–15.
Goode EF, Smyth EC. Immunotherapy for gastroesophageal cancer. J Clin Med. 2016;5(10):84.
Bankfalvi A, Piffko J. Prognostic and predictive factors in oral cancer: the role of the invasive tumour front. J Oral Pathol Med. 2000;29(7):291–8.
Kristensen GB, Abeler VM, Risberg B, Trop C, Bryne M. Tumor size, depth of invasion, and grading of the invasive tumor front are the main prognostic factors in early squamous cell cervical carcinoma. Gynecol Oncol. 1999;74(2):245–51.
Fridman WH, Pages F, Sautes-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer. 2012;12(4):298–306.
Ogino S, Galon J, Fuchs CS, Dranoff G. Cancer immunology: analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8(12):711–9.
Lee HE, Chae SW, Lee YJ, et al. Prognostic implications of type and density of tumour-infiltrating lymphocytes in gastric cancer. Br J Cancer. 2008;99(10):1704–11.
Lee JS, Won HS, Sun S, Hong JH, Ko YH. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a systematic review and meta-analysis. Medicine. 2018;97(32):e11769.
Song HJ, Kim KM. Pathology of epstein-barr virus-associated gastric carcinoma and its relationship to prognosis. Gut Liver. 2011;5(2):143–8.
Eo WK, Jeong DW, Chang HJ, et al. Absolute monocyte and lymphocyte count prognostic score for patients with gastric cancer. World J Gastroenterol. 2015;21(9):2668–76.
Choi Y, Kim JW, Nam KH, et al. Systemic inflammation is associated with the density of immune cells in the tumor microenvironment of gastric cancer. Gastric Cancer. 2017;20(4):602–11.
Hanyuda A, Ogino S, Qian ZR, et al. Body mass index and risk of colorectal cancer according to tumor lymphocytic infiltrate. Int J Cancer. 2016;139(4):854–68.
Wang DL, Liu YY, Gu YL, et al. Increased number of forkhead box P3+ tumor-infiltrating lymphocytes correlates with high preoperative albumin level and better survival in patients with stage II or III colorectal cancer. Tumour Biol. 2015;36(7):5407–14.
Acknowledgment
The authors thank Yuki Orito and Amphone Okada for excellent technical assistance, and Anne M. O’Rourke, PhD, from Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Funding
Part of this study was supported by a Grant-in-Aid for Scientific Research (16K10533) from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
Author information
Authors and Affiliations
Contributions
Study conception and design: YuO, YT, YoO, and MK. Acquisition and analysis of the data: YuO, YT, YoO, AY, CY, KK, YK, TS, SI, TK, HF, HY, JH, SY, and MO. Interpretation of the data: YuO, YT, and YoO. Drafting of the manuscript: YuO, YT, YoO, and MK. Critical revision of the manuscript: YuO, YT, YoO, AY, CY, KK, YK, TS, SI, TK, HF, HY, JH, SY, MO, and MK.
Corresponding authors
Ethics declarations
Yusuke Omura, Yuji Toiyama, Yoshinaga Okugawa, Akira Yamamoto, Chengzeng Yin, Kurando Kusunoki, Yukina Kusunoki, Tsunehiko Shigemori, Shozo Ide, Takahito Kitajima, Hiroyuki Fujikawa, Hiromi Yasuda, Junichiro Hiro, Shigeyuki Yoshiyama, Masaki Ohi, and Masato Kusunoki have no conflicts of interest to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig.
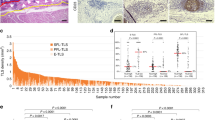
1 Histology of CLR and lymphoid aggregates and CD8+ T-cell infiltration. (a–c) Representative H&E-stained sections of gastric cancer showing LAs not included in the definition of CLR (arrows): (a) LAs associated with mucous membrane; (b) LAs considered parts of the small lymph nodes; and (c) irregularly shaped or long and narrow non-nodular LAs. (d) Immunohistochemical staining of CD8+ T lymphocytes (red) in the tumor margin. Original magnification × 100. CLR Crohn’s-like lymphoid reaction, H&E hematoxylin and eosin, LAs lymphoid aggregates (TIFF 7235 kb)
Supplementary Fig.
2 Kaplan–Meier analysis of patient survival according to CLR size. (a–d) Kaplan–Meier (a, b) overall survival curves and (c, d) disease-free survival for GC patients, stratified based on the median maximum CLR diameter in normal mucosa (a, c: 0 mm) and gastric cancer (b, d: 0.57 mm). (e) Cancer-specific survival stratified by CLR size for 70 patients with pStage II disease and 91 patients with pStage III disease. CLR Crohn’s-like lymphoid reaction (TIFF 853 kb)
Supplementary Fig.
3 Correlation between peritumoral CD8+ T-cell count, CLR size, and patient survival. (a, b) Correlation between CD8+ T-cell count in the tumor margin and CLR size (p < 0.0001, R = 0.66). (c–f) Kaplan–Meier survival curves for (c) cancer-specific survival, (d) recurrence-free survival, (e) overall survival, and (f) disease-free survival in GC patients based on a cut-off value of median CD8+ T cells present in the tumor margin. CLR Crohn’s-like lymphoid reaction, GC gastric cancer (TIFF 945 kb)
Rights and permissions
About this article
Cite this article
Omura, Y., Toiyama, Y., Okugawa, Y. et al. Crohn’s-Like Lymphoid Reaction is Associated with Oncological Prognosis and Nutritional Status in Patients with Pathological Stage II/III Gastric Cancer. Ann Surg Oncol 27, 259–267 (2020). https://doi.org/10.1245/s10434-019-07709-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07709-w