Abstract
Background
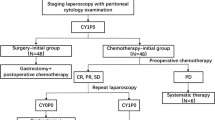
Gastric cancer (GC) patients with positive peritoneal lavage cytology (CY1) and/or localized peritoneum metastasis (P1a) are defined as stage IV in the 15th edition of the Japanese Classification of Gastric Cancer. In Japan, the most common treatment for patients with CY1 and/or P1a is gastrectomy followed by postoperative chemotherapy.
Patients and Methods
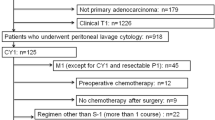
Subjects in this multi-institutional retrospective study were GC patients with CY1 and/or P1a who received surgical resection that leaves no macroscopically visible disease. Patients were selected from 34 institutions in Japan between 2007 and 2012. Selection criteria included adenocarcinoma, no distant metastasis except CY1 and P1a, and no prior treatment for GC before surgery.
Results
Among 824 patients registered, 506 were identified as eligible, with a background of P0CY1, P1aCY0, or P1aCY1 (72.5%, 16.0%, and 11.5% of subjects, respectively). Sixty-two patients had not received postoperative chemotherapy (no-Cx), whereas 444 patients had received postoperative chemotherapy: S-1 monotherapy (S-1; n = 267, 52.7%), cisplatin plus S-1 (CS; n = 114, 22.5%), and others (n = 63, 12.6%). Overall survival (OS) was 29.5, 24.7, 25.4 and 9.9 months in the S-1, CS, ‘others’, and no-Cx groups, respectively [CS vs. S-1: hazard ratio (HR) 1.15, 95% confidence interval (CI) 0.89–1.50; p = 0.275]. In multivariate analysis, OS was similar between the S-1 and CS groups (CS vs. S-1: HR 1.19, 95% CI 0.92–1.55; p = 0.18).
Conclusions
Postoperative chemotherapy after gastrectomy that leaves no macroscopically visible disease may have some survival benefits for GC patients with CY1 and/or P1a. In contrast, S-1 plus cisplatin seems to have no additional benefit over S-1 treatment alone.



Similar content being viewed by others
References
International Agency for Research on Cancer. GLOBOCAN 2012: cancer incidence and mortality worldwide. http://globocan.iarc.fr/Default.aspx. Accessed 21 Aug 2018.
Center for Cancer Control and Information Services, National Cancer Canter, Japan. http://ganjoho.ncc.go.jp/public/statistics/pub/statistics06.html.
Bando E, Yonemura Y, Takeshita Y, et al. Intraoperative lavage for cytological examination in 1297 patients with gastric carcinoma. Am J Surg. 1999; 178:256–62.
Fujiwara Y, Doki Y, Taniguchi H, et al. Genetic detection of free cancer cells in the peritoneal cavity of the patient with gastric cancer: present status and future perspectives. Gastric Cancer. 2007;10: 197–204.
Nakajima T, Harashima S, Hirata M, Kajitani T. Prognostic and therapeutic values of peritoneal cytology in gastric cancer. Acta Cytol. 1978; 22:225–29.
Kodera Y, Yamamura Y, Shimizu Y, et al. Peritoneal washing cytology: prognostic value of positive findings in patients with gastric carcinoma undergoing a potentially curative resection. J Surg Oncol. 1999; 72:60–5.
Kodera Y, Nakanishi H, Ito S, Yamamura Y, Fujiwara M, Koike M. Prognostic significance of intraperitoneal cancer cells in gastric carcinoma: detection of cytokeratin 20 mRNA in peritoneal washes, in addition to detection of carcinoembryonic antigen. Gastric Cancer. 2005; 8:142–8.
Burke EC, Karpeh MS Jr, Conlon KC, Brennan MF. Peritoneal lavage cytology in gastric cancer: an independent predictor of outcome. Ann Surg Oncol. 1998; 5:411–5.
Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma, 2nd English edition. Gastric Cancer 1998; 1:10–24.
Sakuramoto S, Sasako M, Yamaguchi T, et al. Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med. 2007;357(18):1810–20.
Kodera Y, Ito S, Mochizuki Y, et al. Long-term follow up of patients who were positive for peritoneal lavage cytologyfinal report from the CCOG0301 study. Gastric Cancer. 2012;15(3):335–7.
Kano Y, Kosugi S, Ishikawa T, et al. Prognostic significance of peritoneal lavage cytology at three cavities in patients with gastric cancer. Surgery. 2015;158(6):1581–9.
Nakayama I, Chin K, Matsushima T, Takahari D, et al. Retrospective comparison of S-1 plus cisplatin versus S-1 monotherapy for the treatment of advanced gastric cancer patients with positive peritoneal cytology but without gross peritoneal metastasis. Int J Clin Oncol. 2017; 22:1060–1068
Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008; 9: 215–21.
Boku N, Yamamoto S, Fukuda H, et al, for the Gastrointestinal Oncology Study Group of the Japan Clinical Oncology Group. Fluorouracil versus combination of irinotecan plus cisplatin versus S-1 in metastatic gastric cancer: a randomised phase 3 study. Lancet Oncol. 2009; 10: 1063–9.
Bang YJ, Kim YW, Yang HK, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): a phase 3 open-label, randomised controlled trial. Lancet. 2012;379(9813):315–21.
Yoshikawa T, Terashima M, Mizusawa J, et al. Four courses versus eight courses of adjuvant S-1 for patients with stage II gastric cancer (JCOG1104 [OPAS-1]): an open-label, phase 3, non-inferiority, randomised trial. Lancet Gastroenterol Hepatol. 2019;4(3):208–16.
Takahari D, Hamaguchi T, Yoshimura K, et al. Feasibility study of adjuvant chemotherapy with S-1 plus cisplatin for gastric cancer. Cancer Chemother Pharmacol. 2011;67(6):1423–8.
Ryu MH, Baba E, Lee KH, et al. Comparison of two different S-1 plus cisplatin dosing schedules as first-line chemotherapy for metastatic and/or recurrent gastric cancer: a multicenter, randomized phase III trial (SOS). Ann Oncol. 2015;26(10):2097–101.
Iwasaki Y, Terashima M, Mizusawa J, et al. Randomized phase III trial of gastrectomy with or without neoadjuvant S-1 plus cisplatin for type 4 or large type 3 gastric cancer: Japan Clinical Oncology Group study (JCOG0501). 2018 ASCO annual meeting. J Clin Oncol. 2018; 36 Suppl: abstract no. 4046.
Cunningham D, Allum WH, Stenning SP, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355(1):11–20.
Smalley SR, Benedetti JK, Haller DG, et al. Updated analysis of SWOG-directed intergroup study 0116: a phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30(19):2327–33.
Tsuburaya A, Yoshida K, Kobayashi M, et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): a phase 3 factorial randomised controlled trial. Lancet Oncol. 2014;15(8):886–93
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006; 24:4991–7
Ajani JA, Rodriguez W, Bodoky G, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: the FLAGS trial. J Clin Oncol. 2010; 28:1547–53
Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–97
Ziyu Li, Zhemin Li, Shuqin Jia, et al. Depth of tumor invasion and tumor-occupied portions of stomach are predictive factors of intra-abdominal metastasis. Chin J Cancer Res. 2017; 29(2): 109–17.
Manzanedo I, Pereira F, Rihuete Caro, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for gastric cancer with peritoneal carcinomatosis: multicenter study of spanish group of peritoneal oncologic surgery (GECOP). Ann Surg Oncol. 2019;26(8):2615–21.
Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015; 26:141–8
Yoshida K, Kodera Y, Kochi M, et al. Addition of docetaxel to oral fluoropyrimidine improves efficacy in patients with Stage III gastric cancer: interim analysis of JACCRO GC-07, a randomized controlled trial. J Clin Oncol. 2019;37(15):1296–304.
Ishigami H, Fujiwara Y, Fukushima R, et al. Phase III trial comparing intraperitoneal and intravenous paclitaxel plus S-1 versus cisplatin plus S-1 in patients with gastric cancer with peritoneal metastasis: PHOENIX-GC trial. J Clin Oncol. 2018;36(19):1922–9.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
Toshifumi Yamaguchi, Atsuo Takashima, Kengo Nagashima, Rie Makuuchi, Masaki Aizawa, Manabu Ohashi, Keitaro Tashiro, Tatsuya Yamada, Takahiro Kinoshita, Hiroaki Hata, Yasuyuki Kawachi, Ryohei Kawabata, Toshikatsu Tsuji, Jun Hihara, Takeshi Sakamoto, Takeo Fukagawa, Hitoshi Katai, Kazuhide Higuchi, and Narikazu Boku have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yamaguchi, T., Takashima, A., Nagashima, K. et al. Efficacy of Postoperative Chemotherapy After Resection that Leaves No Macroscopically Visible Disease of Gastric Cancer with Positive Peritoneal Lavage Cytology (CY1) or Localized Peritoneum Metastasis (P1a): A Multicenter Retrospective Study. Ann Surg Oncol 27, 284–292 (2020). https://doi.org/10.1245/s10434-019-07697-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07697-x