Abstract
Background
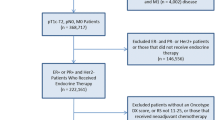
Uncertainty regarding chemotherapy benefit among breast cancer patients with intermediate Oncotype Dx® recurrence scores (RS; 11–25) led to the TAILORx study. We evaluated chemotherapy use in patients with intermediate RS to determine practice change potential based on the TAILORx results.
Methods
National Cancer Data Base patients with hormone receptor-positive/human epidermal growth factor receptor 2 (HER2)-negative, N0 breast cancer were identified and were divided into three groups: Group A, ≤ 50 years of age (RS 11–15); Group B, ≤ 50 years of age (RS 16–25); and Group C, > 50 years of age (RS 11–25). Demographic and clinical factors were compared using Chi square tests and Poisson regression models to determine predictors of chemotherapy receipt.
Results
Overall, 37,087 patients met the inclusion criteria, with 6.3% in Group A and 11.7% in Group C having received chemotherapy that may have been avoided based on TAILORx. The majority of Group B (64.7%) did not receive chemotherapy, whereas TAILORx showed potential benefit from treatment. Chemotherapy use decreased over time for all intermediate RS patients. T2 tumors, high grade, and treatment before 2012 increased the likelihood of chemotherapy receipt among both groups. Younger patients with the lower intermediate RS (Group A) were more likely to receive chemotherapy if they had treatment at community or comprehensive centers, whereas moderate grade was also a significant factor to receive chemotherapy in Group B. Significant factors in older patients (Group C) were Black race, estrogen receptor-positive/progesterone receptor-negative, and moderate/high grade.
Conclusions
The most potential impact of TAILORx findings on practice change is for patients ≤ 50 years of age with RS of 16–25 who did not receive chemotherapy but may benefit. These findings may serve as a baseline for future analysis of practice patterns related to TAILORx.


Similar content being viewed by others
References
National Comprehensive Cancer Network. Evidence-based cancer guidelines, oncology drug compendium, oncology continuing medical education. https://www.nccn.org/. Accessed 1 Jun 2019.
Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–26.
Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65.
Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28(11):1829–34.
Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–21.
Cronin M, Sangli C, Liu ML, et al. Analytical validation of the Oncotype Dx genomic diagnostic test for recurrence prognosis and therapeutic response prediction in node-negative, estrogen receptor-positive breast cancer. Clin Chem. 2007;53(6):1084–91.
Sparano JA, Paik S. Development of the 21-gene assay and its application in clinical practice and clinical trials. J Clin Oncol. 2008;26(5):721–8.
Sparano JA, Gray RJ, Makower DF, et al. Prospective validation of a 21-Gene expression assay in breast cancer. N Engl J Med. 2015;373(21):2005–14.
Bhutiani N, Egger ME, Ajkay N, Scoggins CR, Martin RC 2nd, McMasters KM. Multigene signature panels and breast cancer therapy: patterns of use and impact on clinical decision making. J Am Coll Surg. 2018;226(4):406–12.e1.
Williams AD, Reyes SA, Arlow RL, Tchou J, De La Cruz LM. Is age trumping genetic profiling in clinical practice? Relationship of chemotherapy recommendation and Oncotype Dx recurrence score in patients aged <50 years versus ≥50 years, and trends over time. Ann Surg Oncol. 2018;25(10):2875–83.
Enewold L, Geiger AM, Zujewski J, Harlan LC. Oncotype Dx assay and breast cancer in the United States: usage and concordance with chemotherapy. Breast Cancer Res Treat. 2015;151(1):149–56.
American College of Surgeons. About the National Cancer Database. https://www.facs.org/quality-programs/cancer/ncdb/about. Accessed 15 Jan 2018.
Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83.
Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–51.
Jasem J, Amini A, Rabinovitch R, et al. 21-Gene recurrence score assay as a predictor of adjuvant chemotherapy administration for early-stage breast cancer: an analysis of use, therapeutic implications, and disparity profile. J Clin Oncol. 2016;34(17):1995–2002.
Ibraheem AF, Press DJ, Olopade OI, Huo D. Community clinical practice patterns and mortality in patients with intermediate Oncotype Dx recurrence scores: who benefits from chemotherapy? Cancer. 2019;125(2):213–22.
Carlson JJ, Roth JA. The impact of the Oncotype Dx breast cancer assay in clinical practice: a systematic review and meta-analysis. Breast Cancer Res Treat. 2013;141(1):13–22.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–10.
Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 2002;41(3A):151–2 [(discussion 152–153) republished from Histopathology . 1991;19:403–10].
Rakha EA, El-Sayed ME, Lee AH, et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 2008;26(19):3153–8.
Mook S, Schmidt MK, Rutgers EJ, et al. Calibration and discriminatory accuracy of prognosis calculation for breast cancer with the online Adjuvant! program: a hospital-based retrospective cohort study. Lancet Oncol. 2009;10(11):1070–6.
Goldhirsch A, Ingle JN, Gelber RD, et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol. 2009;20(8):1319–29.
Fisher B, Jeong JH, Dignam J, et al. Findings from recent National Surgical Adjuvant Breast and Bowel Project adjuvant studies in stage I breast cancer. J Natl Cancer Inst Monogr. 2001;(30):62–6.
Mansour EG, Gray R, Shatila AH, et al. Survival advantage of adjuvant chemotherapy in high-risk node-negative breast cancer: ten-year analysis—an intergroup study. J Clin Oncol. 1998;16(11):3486–92.
National Institutes of Health Consensus Development Panel. National Institutes of Health Consensus Development Conference statement: adjuvant therapy for breast cancer, November 1–3, 2000. J Natl Cancer Inst Monogr. 2001;(30):5–15.
Orucevic A, Bell JL, King M, McNabb AP, Heidel RE. Nomogram update based on TAILORx clinical trial results—Oncotype Dx breast cancer recurrence score can be predicted using clinicopathologic data. Breast. 2019;46:116–25.
Singh K, He X, Kalife ET, Ehdaivand S, Wang Y, Sung CJ. Relationship of histologic grade and histologic subtype with Oncotype Dx recurrence score; retrospective review of 863 breast cancer Oncotype Dx results. Breast Cancer Res Treat. 2018;168(1):29–34.
Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff (Millwood). 2002;21(2):60–76.
Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993;329(5):326–31.
Freedman RA, Virgo KS, He Y, et al. The association of race/ethnicity, insurance status, and socioeconomic factors with breast cancer care. Cancer. 2011;117(1):180–9.
Harlan LC, Greene AL, Clegg LX, Mooney M, Stevens JL, Brown ML. Insurance status and the use of guideline therapy in the treatment of selected cancers. J Clin Oncol. 2005;23(36):9079–88.
Wu XC, Lund MJ, Kimmick GG, et al. Influence of race, insurance, socioeconomic status, and hospital type on receipt of guideline-concordant adjuvant systemic therapy for locoregional breast cancers. J Clin Oncol. 2012;30(2):142–50.
Dinan MA, Mi X, Reed SD, Hirsch BR, Lyman GH, Curtis LH. Initial trends in the use of the 21-gene recurrence score assay for patients with breast cancer in the medicare population, 2005–2009. JAMA Oncol. 2015;1(2):158–66.
Guth AA, Fineberg S, Fei K, Franco R, Bickell NA. Utilization of Oncotype Dx in an inner city population: race or place? Int J Breast Cancer. 2013;2013:653805.
Roberts MC, Weinberger M, Dusetzina SB, et al. Racial variation in the uptake of Oncotype Dx testing for early-stage breast cancer. J Clin Oncol. 2016;34(2):130–8.
Sparano JA, Gray RJ, Ravdin PM, et al. Clinical and genomic risk to guide the use of adjuvant therapy for breast cancer. N Engl J Med. 2019;380(25):2395–405.
Mariotto A, Jayasekerea J, Petkov V, et al. Expected monetary impact of Oncotype Dx score-concordant systemic breast cancer therapy based on the TAILORx trial. J Natl Cancer Inst. https://doi.org/10.1093/jnci/djz068.
Acknowledgment
The authors wish to acknowledge the support of the Biostatistics Shared Resource Facility and National Cancer Institute Cancer Center Support Grant P30 CA196521-01, Icahn School of Medicine at Mount Sinai, for analysis and interpretation of data and preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
LDLC conceptualized the study; LDLC and EP designed the study; LDLC and SR drafted the initial manuscript; and MR and KP performed data collection and analysis. All authors reviewed, revised, and approved the final manuscript as submitted, and agree to be accountable for all aspects of this work.
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Reyes, S.A., De La Cruz, L.M., Ru, M. et al. Practice Changing Potential of TAILORx: A Retrospective Review of the National Cancer Data Base from 2010 to 2015. Ann Surg Oncol 26, 3397–3408 (2019). https://doi.org/10.1245/s10434-019-07650-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07650-y