Abstract
Background
Sentinel lymph node biopsy (SLNB) is increasingly utilized after neoadjuvant chemotherapy (NAC) in responsive adenopathy, particularly with placement of a marking clip in the involved node(s). This may allow a subset of patients to avoid axillary lymph node dissection. SLNB is still discouraged in inflammatory breast cancer (IBC). The purpose of this study is to examine the axillary pathologic complete response (AXpCR) in IBC patients with clinical adenopathy. There may be an implication to approach a subset of IBC patients for SLNB after NAC.
Methods
A single-institution institutional review board-approved database was reviewed. Inclusion criteria were clinicopathologic diagnosis of IBC and age ≥ 18 years. Stage IV disease was excluded. We collected data on demographics, tumor characteristics including histology and subtype, axillary status, and treatment effect details.
Results
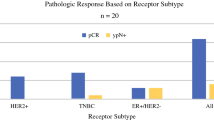
Sixty-six patients fulfilled criteria. Mean follow-up was 4.1 years. The AXpCR was 6% for luminal A and luminal B [human epidermal growth factor receptor (HER)2 −] subtypes, and 24% for basal subtype. The AXpCR rate was 64% for HER2-enriched and luminal B (HER2 +) patients. Achievement of AXpCR among these HER2-positive patients was statistically significant (p = 0.0001). There was minimal difference in achieving AXpCR in HER2-overexpressing patients regardless of hormone receptor status (p = 1.000).
Conclusions
Understanding the best patients to select for use of SLNB or targeted lymph node dissection after treatment is evolving. This unique series identified and described the axillary pathologic characteristics of IBC patients following NAC. Further research is needed to confirm that the approach, axillary node clip placement prior to treatment, is feasible and accurate in IBC.

Similar content being viewed by others
References
Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–933.
Veronesi U, Giuseppe V, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251:595–600.
Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: The American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–432.
Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol. 1999; 17(2):460–469.
Fayanju OM, Ren Y, Thomas SM, et al. The clinical significance of breast-only and node-only pathologic complete response (pCR) after neoadjuvant chemotherapy (NACT): a review of 20,000 breast cancer patients in the National Cancer Data Base (NCDB). Ann Surg. 2018; 268(4):591–601.
Liedtke C, Mazouni C, Hess KR, et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J Clin Oncol. 2008; 26(8):1275–1281.
Cortazar P, Zhang L, Untch M, et al. Pathologic complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014; 384:164–167.
von Minckwitz G, Untch M, Biohmer JU, et al. Definition and impact of pathologic complete response on prognosis after neoadjuvant chemotherapy in various intrinsic breast cancer subtypes. J Clin Oncol. 2012; 30(15):1796–1804.
Ueno NT, Buzdar AU, Singletary SE, et al. Combined-modality treatment in inflammatory breast carcinoma: twenty years of experience at M.D. Anderson Cancer Center. Cancer Chemother Pharmacol. 1997;40:321–329.
Cristofanilli M, Gonzalez-Angulo AM, Buzdar AU, et al. Paclitaxel improves the prognosis in estrogen receptor negative inflammatory breast cancer: the M. D. Anderson Cancer Center experience. Clin Breast Cancer. 2004;4:415–419.
Gianni L, Pienkowski T, Young-Hyuck I, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advance, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicenter, open label, phase 2 trial. Lancet Oncol. 2012;13:25–32.
Gianni L, Pienkowski T, Young-Hyuck I, et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): a multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016;17:791–800.
Ogawa L, Lindquist D. Dual HER2 Suppression with lapatinib plus trastuzumab for metastatic inflammatory breast cancer: a case report of prolonged stable disease. Case Rep Oncol. 2018;11:855–860.
Dawood S, Merajver SD, Viens P, et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22:515–523.
Stearns V, Ewing CA, Slack R, et al. Sentinel lymphadenectomy after neoadjuvant chemotherapy for breast cancer may reliably represent the axilla except for inflammatory breast cancer. Ann Surg Oncol. 2002;9(3):235–242.
Hidar S, Bibi M, Gharbi O, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy in inflammatory breast cancer. Int J Surg. 2009;7:272–275.
Caudle AS, Yang WT, Krishnamurthy S, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016;34:1072–1078.
Boughey JC, Suman VJ, Mittendorf EA, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The American College of Surgeons Oncology Group (ACOSOG) Z1071 clinical trial. JAMA. 2013;310(14):1455–1461.
Nguyen TT, Hoskin TL, Day CN, et al. Decreasing use of axillary dissection in node-positive breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Onc. 2018;25:2596–2602.
Liu J, Chen K, Jiang W, et al. Chemotherapy response and survival of inflammatory breast cancer by hormone receptor- and HER2-defined molecular subtypes approximation: an analysis from the National Cancer Database. J Cancer Res Clin Oncol. 2017;143:161–168.
Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ASCOG Z1071 (Alliance) prospective multicenter clinical trial. Ann Surg. 2014;260(4):608–616.
Kuehn T, Bauerfeind I, Fehm T, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol. 2013;14(7):609–618.
Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC Study. J Clin Oncol. 2015;33(3):258–264.
Mamtani A, Barrio AV, King TA, et. al. How often does neoadjuvant chemotherapy avoid axillary dissection in patients with histologically confirmed nodal metastases? Results of a prospective study. Ann Surg Oncol. 2016;23(11):3467–3474.
DeSnyder SM, Mittendorf EA, Le-Petross C, et al. Prospective feasibility trial of sentinel lymph node biopsy in the setting of inflammatory breast cancer. Clin Breast Cancer. 2018;18(1):e73–e77.
Krag DN and Single RM. Breast cancer survival according to number of nodes removed. Ann Surg Oncol. 2003;10(10):1152–1159.
Bilimoria KY, Bentrem DJ, Hansen NM, et al. Comparison of sentinel lymph node biopsy alone and completion axillary lymph node dissection for node-positive breast cancer. J Clin Oncol. 2009;27(18):2946–2953.
Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
The authors have no disclosures of any commercial interest in the subject of study or relevant financial or material support.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Imeokparia, F.O., Hughes, T.M., Dossett, L.A. et al. Axillary Pathologic Complete Response in Inflammatory Breast Cancer Patients: Implications for SLNB?. Ann Surg Oncol 26, 3374–3379 (2019). https://doi.org/10.1245/s10434-019-07597-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07597-0