Abstract
Background
The utilization of OncotypeDx in the setting of neoadjuvant chemotherapy (NCT) is not well defined. The objective of this study was to determine what proportion of hormone receptor (HR)-positive patients undergoing NCT would not benefit from chemotherapy based on OncotypeDx recurrence scores (RS) and predictors of a high RS as defined by the TAILORx trial.
Methods
The National Cancer Data Base was used to identify patients with unilateral clinical stage I–III HR+/Her2− breast cancer who had an OncotypeDx score and who had undergone NCT. Patients undergoing adjuvant chemotherapy were used as a comparison group.
Results
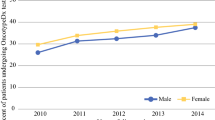
Of 307,666 patients, 41.8% had testing with OncotypeDx. Of these, 76.6% had no chemotherapy, 22.3% adjuvant chemotherapy, and 1.1% NCT. OncotypeDx testing in NCT patients increased from 4.9% in 2010 to 8.2% in 2015. Of NCT patients with OncotypeDx testing, 11.6% had RS < 11, 44.4% RS 11–25, and 43.9% RS > 25. In patients age ≤ 50 years, 14.5% had RS < 11, 12.4% RS 11–15, 31.4% RS 16–25, and 41.7% RS > 25. Predictors of RS > 25 on multivariable analysis included grade 3 tumors (odds ratio [OR] 3.83) and PR-negative tumors (OR 5.26) but not clinical T or N stage (p > 0.05).
Conclusions
More than half of patients with OncotypeDx testing are being overtreated with NCT, and a third of younger patients are being overtreated. Predictors of a high RS are reliably available at core biopsy, suggesting an application of OncotypeDx in determining the need for NCT for some HR-positive breast cancers.


Similar content being viewed by others
References
Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–26.
Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–34.
Dowsett M, Cuzick J, Wale C, et al. Prediction of risk of distant recurrence using the 21-gene recurrence score in node-negative and node-positive postmenopausal patients with breast cancer treated with anastrozole or tamoxifen: a TransATAC study. J Clin Oncol. 2010;28(11):1829–34.
Albain KS, Barlow WE, Shak S, et al. Prognostic and predictive value of the 21-gene recurrence score assay in postmenopausal women with node-positive, oestrogen-receptor-positive breast cancer on chemotherapy: a retrospective analysis of a randomised trial. Lancet Oncol. 2010;11(1):55–65.
Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–21.
National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version 1. 2019. https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed 14 April 2019.
American Joint Committee on Cancer. AJCC cancer staging manual, 8th edn. Chicago, IL: Springer; 2017.
Bhutiani N, Egger ME, Ajkay N, Scoggins CR, Martin RC 2nd, McMasters KM Multigene signature panels and breast cancer therapy: patterns of use and impact on clinical decision making. J Am Coll Surg. 2018;226(4):406–12.
Anderson J, Shak S, Millward C, Labusch M, Chen I, Watson D, Baehner F. Molecular characterization of breast cancer core biopsy specimens by gene expression analysis using standardized quantitative RT-PCR. Cancer Res. 2009;69(24 suppl):6021.
Gianni L, Zambetti M, Clark M, et al. Gene expression profiles in paraffin-embedded core biopsy tissue predict response to chemotherapy in women with locally advanced breast cancer. J Clin Oncol. 2005;23(29):7265–77.
Chang JC, Makris A, Gutierrez MC, et al. Gene expression patterns in formalin-fixed, paraffin-embedded core biopsies predict docetaxel chemosensitivity in breast cancer patients. Breast Cancer Res Treat. 2008;108(2):233–40.
Yardley DA, Peacock NW, Shastry M, et al. A phase II trial of ixabepilone and cyclophosphamide as neoadjuvant therapy for patients with Her2-negative breast cancer: correlation of pathologic complete response with the 21-gene recurrence score. Breast Cancer Res Treat. 2015;154(2):299–308.
Soran A, Bhargava R, Johnson R, et al. The impact of OncotypeDx® recurrence score of paraffin-embedded core biopsy tissues in predicting response to neoadjuvant chemotherapy in women with breast cancer. Breast Dis. 2016;36(2–3):65–71.
Bear HD, Wan W, Robidoux A, et al. Using the 21-gene assay from needle biopsies to choose neoadjuvant therapy for breast cancer: a multicenter trial. J Surg Oncol. 2017;115(8):917–23.
Iwata H, Masuda N, Yamamoto Y, et al. Validation of the 21-gene test as a predictor of clinical response to neoadjuvant hormonal therapy for ER+, Her2-negative breast cancer: the TransNEOS study. Breast Cancer Res Treat. 2019;173(1):123–33.
Bilimoria KY, Bentrem DJ, Stewart AK, Winchester DP, Ko CY. Comparison of commission on cancer-approved and -nonapproved hospitals in the United States: implications for studies that use the National Cancer Data Base. J Clin Oncol. 2009;27:4177–81.
American Joint Committee on Cancer. AJCC cancer staging manual, 7th edn. Chicago, IL: Springer; 2010.
Pease AM, Riba LA, Gruner RA, Tung NM, James TA. OncotypeDx® recurrence score as a predictor of response to neoadjuvant chemotherapy. Ann Surg Oncol. 2019;26(2):366–71.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kantor, O., Barrera, E., Kopkash, K. et al. Are we Overtreating Hormone Receptor Positive Breast Cancer with Neoadjuvant Chemotherapy? Role of OncotypeDx® for Hormone Receptor Positive Patients Undergoing Neoadjuvant Chemotherapy. Ann Surg Oncol 26, 3232–3239 (2019). https://doi.org/10.1245/s10434-019-07555-w
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07555-w