Abstract
Background
Recurrence score (RS) testing in early-stage, ER-positive breast cancer is used to predict the benefit of adjuvant chemotherapy for disease recurrence and overall survival. TAILORx results decreased the ambiguity of “intermediate risk” RS by creating a binary classification system. We aimed to determine how women ≥ 70 years with intermediate RS were redistributed post-TAILORx and to identify predictors of low RS.
Methods
Patients ≥ 70 years with early-stage, node-negative, ER-positive breast cancers in the National Cancer Database(2006–2014) were included. “Pre-TAILORx” RS were classified as low (0–17), intermediate (18–30), and high (> 30). “Post-TAILORx” RS were classified as low (0–25) and high (> 25).
Results
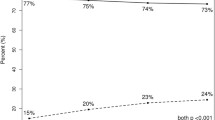
In total, 14,925 women were included. Average age was 74 years. 60% (n = 9009) had low pre-TAILORx RS, 31% (n = 4635) intermediate, and 9% (n = 1281) high. Of 4635 patients with intermediate RS, 72% (n = 3660) were reclassified to low RS. Only 12% (n = 1783) of patients received chemotherapy. Of patients with pre-TAILORx intermediate RS who received chemotherapy, 55% (n = 417) would have been spared chemotherapy by being reclassified with low RS post-TAILORx. The strongest predictor of post-TAILORx low RS was tumor grade; 95% of well-differentiated had low RS, compared with 56% of poorly/undifferentiated tumors (p < 0.001). Smaller tumor size also was associated with low RS. Age was not associated with RS.
Conclusions
With post-TAILORx RS criteria, the vast majority of patients ≥ 70 years can be classified as low-risk and unlikely to benefit from chemotherapy. Given that the elderly have greater rates of chemotherapy-associated complications, reconsideration of routine RS testing in patients ≥ 70 years is warranted. Tumor grade and size also may inform the decision to omit RS testing.

Similar content being viewed by others
References
Fisher B, Costantino J, Redmond C, et al. A randomized clinical trial evaluating tamoxifen in the treatment of patients with node-negative breast cancer who have estrogen-receptor-positive tumors. N Engl J Med. 1989;320(8):479–84.
Fisher B, Dignam J, Wolmark N, et al. Tamoxifen and chemotherapy for lymph node-negative, estrogen receptor-positive breast cancer. J Natl Cancer Inst. 1997;89(22):1673–82.
Fisher B, Jeong JH, Bryant J, et al. Treatment of lymph-node-negative, oestrogen-receptor-positive breast cancer: long-term findings from National Surgical Adjuvant Breast and Bowel Project randomised clinical trials. Lancet. 2004;364(9437):858–68.
Paik S, Shak S, Tang G, et al. A multigene assay to predict recurrence of tamoxifen-treated, node-negative breast cancer. N Engl J Med. 2004;351(27):2817–26.
Sparano JA, Gray RJ, Makower DF, et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med. 2018;379(2):111–21.
Muss HB. Adjuvant chemotherapy in older women with breast cancer: who and what? J Clin Oncol. 2014;32(19):1996–2000.
Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–90.
Nickleach D, Liu Y, A S. SAS (R) macros to conduct common biostatistical analyses and generate reports. SESUG 2013: The Proceeding of the SouthEast SAS User Group. 2013.
Partin JF, Mamounas EP. Impact of the 21-gene recurrence score assay compared with standard clinicopathologic guidelines in adjuvant therapy selection for node-negative, estrogen receptor-positive breast cancer. Ann Surg Oncol. 2011;18(12):3399–406.
Harris L, Fritsche H, Mennel R, et al. American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–312.
National Comprehensive Cancer Network: Clinical Practice Guidelines in Oncology Breast Cancer (Version 2.2008) http://www.nccn.org/professionals/physician_gls/f/guidelines.asp.
Hortobagyi G, Connolly J, D’Orsi C, et al. Breast. In: Amin M, ed. AJCC Cancer Staging Manual Eighth Edition. Chicago, IL: Springer; 2017:589–628.
Paik S, Tang G, Shak S, et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol. 2006;24(23):3726–34.
Diab S, Elledge R, Clark G. Tumor characteristics and clinical outcome of elderly women with breast cancer. J Natl Cancer Inst. 2000;92(7):550–6.
Gennari R, Curigliano G, Rotmensz N, et al. Breast carcinoma in elderly women: features of disease presentation, choice of local and systemic treatments compared with younger postmenopasual patients. Cancer. 2004;101(6):1302–10.
Hughes KS, Schnaper LA, Bellon JR, et al. Lumpectomy plus tamoxifen with or without irradiation in women age 70 years or older with early breast cancer: long-term follow-up of CALGB 9343. J Clin Oncol. 2013;31(19):2382–7.
Takuwa H, Tsuji W, Yotsumoto F. Overall survival of elderly patients with breast cancer is not related to breast-cancer specific survival: A single institution experience in Japan. Breast Dis. 2018;37(4):177–83.
Li S, Liu J, Virnig BA, Collins AJ. Association between adjuvant chemotherapy and risk of acute kidney injury in elderly women diagnosed with early-stage breast cancer. Breast Cancer Res Treat. 2017;161(3):515–24.
Srokowski TP, Fang S, Hortobagyi GN, Giordano SH. Impact of diabetes mellitus on complications and outcomes of adjuvant chemotherapy in older patients with breast cancer. J Clin Oncol. 2009;27(13):2170–6.
Barcenas CH, Niu J, Zhang N, et al. Risk of hospitalization according to chemotherapy regimen in early-stage breast cancer. J Clin Oncol. 2014;32(19):2010–7.
Hoogerduijn JG, Buurman BM, Korevaar JC, Grobbee DE, de Rooij SE, Schuurmans MJ. The prediction of functional decline in older hospitalised patients. Age Ageing. 2012;41(3):381-7.
Kunkler IH, Williams LJ, Jack WJ, Cameron DA, Dixon JM, investigators PI. Breast-conserving surgery with or without irradiation in women aged 65 years or older with early breast cancer (PRIME II): a randomised controlled trial. Lancet Oncol. 2015;16(3):266–73.
Acknowledgments
Research reported in this publication was supported in part by the Biostatistics and Bioinformatics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award number P30CA138292. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The data used in the study are derived from a de-identified NCDB file. The American College of Surgeons and the Commission on Cancer have not verified and are not responsible for the analytic or statistical methodology employed, or the conclusions drawn from these data by the investigator.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
None.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, R.M., Switchenko, J.M., Ho, T.B. et al. Is Routine Recurrence Score Testing in Patients Older than 70 Years of Age Warranted? An Evaluation of the National Cancer Database After TAILORx. Ann Surg Oncol 26, 3152–3158 (2019). https://doi.org/10.1245/s10434-019-07553-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07553-y