Abstract
Background
Surgery after neoadjuvant chemotherapy (NCT) is an accepted treatment approach for locally advanced and some early-stage breast cancers, even for patients with a clinical complete response (cCR) after NCT. This study sought to evaluate the survival outcomes for patients with cCR to NCT who did not undergo surgery.
Methods
The National Cancer Data Base (NCDB) was used to identify 93,417 women age 18 years or older with a diagnosis of invasive breast cancer who received NCT between 2010 and 2015. The study identified 350 women with cT1-4, N0-3, and M0 tumors who underwent NCT and did not have surgery. A matched surgical cohort was extracted from the NCDB, and overall survival (OS) was compared between the surgical and nonsurgical patients after NCT.
Results
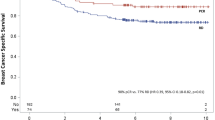
Of the 350 NCT patients who did not undergo surgery, 45 (12.9%) had cCR, 51 (14.6%) had a partial response, 241 (68.9%) had a response but whether complete or partial was not recorded, and 13 (3.7%) had no response/progression. The 5-year OS was better in the cCR group than in the no-cCR group (96.8% vs 69.8%; p = 0.004). A 5-year OS analysis of the cCR patients without surgery (n = 45; median follow-up period, 37 months) compared with the patients with a pathologic complete response who underwent surgery (n = 3938; median follow-up period, 43 months) showed no statistically significant difference (96.8% vs 92.5%, respectively; p = 0.15).
Conclusion
This retrospective cohort study demonstrated that active surveillance or de-escalation therapy may be an option for patients who achieve cCR. Prospective studies are underway to determine whether a subgroup of patients may forgo surgery in the setting of cCR after NCT.




Similar content being viewed by others
References
Criscitiello C, Golshan M, Barry WT, Viale G, Wong S, Santangelo M, Curigliano G. Impact of neoadjuvant chemotherapy and pathological complete response on eligibility for breast-conserving surgery in patients with early breast cancer: a meta-analysis. Eur J Cancer. 2018;97:1–6.
Choi J, Laws A, Hu J, Barry W, Golshan M, King T. Margins in breast-conserving surgery after neoadjuvant therapy. Ann Surg Oncol. 2018;25:3541–7.
Rea D, Tomlins A, Francis A. Time to stop operating on breast cancer patients with pathological complete response? Eur J Surg Oncol. 2013;39:924–30.
van la Parra RF, Kuerer HM. Selective elimination of breast cancer surgery in exceptional responders: historical perspective and current trials. Breast Cancer Res. 2016;18:28.
Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol. 1998;16:2672–85.
Schneeweiss A, Chia S, Hickish T, et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA). Ann Oncol. 2013;24:2278–84.
Houssami N, Macaskill P, von Minckwitz G, Marinovich ML, Mamounas E. Meta-analysis of the association of breast cancer subtype and pathologic complete response to neoadjuvant chemotherapy. Eur J Cancer. 2012;48:3342–54.
Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–72.
Heil J, Sinn P, Richter H, et al. RESPONDER: diagnosis of pathological complete response by vacuum-assisted biopsy after neoadjuvant chemotherapy in breast cancer: a multicenter, confirmative, one-armed, intra-individually-controlled, open, diagnostic trial. BMC Cancer. 2018;18:851.
Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173:676–82.
Chen Y, Shi XE, Tian JH, Yang XJ, Wang YF, Yang KH. Survival benefit of neoadjuvant chemotherapy for resectable breast cancer: a meta-analysis. Medicine Baltimore. 2018;97:e10634.
Li X, Dai D, Chen B, Tang H, Wei W. Oncological outcome of complete response after neoadjuvant chemotherapy for breast-conserving surgery: a systematic review and meta-analysis. World J Surg Oncol. 2017;15:210.
Guidance for Industry Pathological Complete Response in Neoadjuvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval. U.S. Department of Health and Human Services Food and Drug Administration, 2014. Retrieved December 2, 2018 from http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm305501.pdf.
Sikov WM, Berry DA, Perou CM, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance). J Clin Oncol. 2015;33:13–21.
Kuerer HM, Rauch GM, Krishnamurthy S, et al. A clinical feasibility trial for identification of exceptional responders in whom breast cancer surgery can be eliminated following neoadjuvant systemic therapy. Ann Surg. 2018;267:946–51.
Deo SV, Bhutani M, Shukla NK, Raina V, Rath GK, Purkayasth J. Randomized trial comparing neoadjuvant versus adjuvant chemotherapy in operable locally advanced breast cancer (T4b N0-2 M0). J Surg Oncol. 2003;84:192–7.
Kuerer HM, Vrancken Peeters M, Rea DW, Basik M, De Los Santos J, Heil J. Nonoperative management for invasive breast cancer after neoadjuvant systemic therapy: conceptual basis and fundamental international feasibility clinical trials. Ann Surg Oncol. 2017;24:2855–62.
Schaefgen B, Mati M, Sinn HP, et al. Can routine imaging after neoadjuvant chemotherapy in breast cancer predict pathologic complete response? Ann Surg Oncol. 2016;23:789–95.
Lobbes MB, Prevos R, Smidt M, et al. The role of magnetic resonance imaging in assessing residual disease and pathologic complete response in breast cancer patients receiving neoadjuvant chemotherapy: a systematic review. Insights Imaging. 2013;4:163–75.
Umphrey H, Bernreuter WK, Bland K, et al. A tri-modality imaging assessment algorithm to evaluate neoadjuvant therapy response in patients with operable breast cancer (abstract). Cancer Res. 2012;72(24 Suppl):abstract nr P3-03.
Eliminating Surgery After Systemic Therapy in Treating Patients With HER2 Positive or Triple Negative Breast Cancer: www.clinicaltrials.gov. Retrieved December 2, 2018 from https://clinicaltrials.gov/ct2/show/NCT02945579.
Towards omitting breast cancer surgery in patients without residual tumor after upfront chemotherapy: Netherlands Trial Register. Retrieved December 2, 2018 from http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=6120.
Francis A, Herring K, Molyneux R, Jafri M, Trivedi S, Shaaban A, Rea DW. NOSTRA PRELIM: a nonrandomised pilot study designed to assess the ability of image-guided core biopsies to detect residual disease in patients with early breast cancer who have received neoadjuvant chemotherapy to inform the design of a planned trial. Cancer Res. 2017;77(4 Suppl):abstract nr P5-16-4.
Assessing the Accuracy of Tumor Biopsies After Chemotherapy to Determine if Patients Can Avoid Breast Surgery: www.clinicaltrials.gov. Retrieved December 2, 2018 from https://clinicaltrials.gov/ct2/show/NCT03188393.
Mamounas EP. Omitting surgery in complete responders after neoadjuvant chemotherapy: the quest continues. Ann Surg Oncol. 2018;25:3119–22.
Acknowledgment
Dr. Enver Özkurt is supported by The Scientific and Technological Research Council of Turkey (TUBITAK) under Grant Number 1059B191700733.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosure
This study was supported by the Breast Cancer Research Foundation, Hale Family Grant.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Özkurt, E., Sakai, T., Wong, S.M. et al. Survival Outcomes for Patients With Clinical Complete Response After Neoadjuvant Chemotherapy: Is Omitting Surgery an Option?. Ann Surg Oncol 26, 3260–3268 (2019). https://doi.org/10.1245/s10434-019-07534-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07534-1