Abstract
Background
Perioperative allogeneic blood transfusion is associated with poor oncologic outcomes in multiple malignancies. The effect of blood transfusion on recurrence and survival in distal cholangiocarcinoma (DCC) is not known.
Methods
All patients with DCC who underwent curative-intent pancreaticoduodenectomy at 10 institutions from 2000 to 2015 were included. Primary outcomes were recurrence-free (RFS) and overall survival (OS).
Results
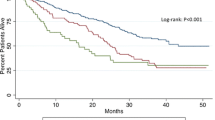
Among 314 patients with DCC, 191 (61%) underwent curative-intent pancreaticoduodenectomy. Fifty-three patients (28%) received perioperative blood transfusions, with a median of 2 units. There were no differences in baseline demographics or operative data between transfusion and no-transfusion groups. Compared with no-transfusion, patients who received a transfusion were more likely to have (+) margins (28 vs 14%; p = 0.034) and major complications (46 vs 16%; p < 0.001). Transfusion was associated with worse median RFS (19 vs 32 months; p = 0.006) and OS (15 vs 29 months; p = 0.003), which persisted on multivariable (MV) analysis for both RFS [hazard ratio (HR) 1.8; 95% confidence interval (CI) 1.1–3.0; p = 0.031] and OS (HR 1.9; 95% CI 1.1–3.3; p = 0.018), after controlling for portal vein resection, estimated blood loss (EBL), grade, lymphovascular invasion (LVI), and major complications. Similarly, transfusion of ≥ 2 pRBCs was associated with lower RFS (17 vs 32 months; p < 0.001) and OS (14 vs 29 months; p < 0.001), which again persisted on MV analysis for both RFS (HR 2.6; 95% CI 1.4–4.5; p = 0.001) and OS (HR 4.0; 95% CI 2.2–7.5; p < 0.001). The RFS and OS of patients transfused 1 unit was comparable to patients who were not transfused.
Conclusion
Perioperative blood transfusion is associated with decreased RFS and OS after resection for distal cholangiocarcinoma, after accounting for known adverse pathologic factors. Volume of transfusion seems to exert an independent effect, as 1 unit was not associated with the same adverse effects as ≥ 2 units.


Similar content being viewed by others
References
Dickson PV, Behrman SW. Distal cholangiocarcinoma. Surg Clin North Am. 2014;94(2):325–42.
Ethun CG, Lopez-Aguiar AG, Pawlik TM, et al. Distal cholangiocarcinoma and pancreas adenocarcinoma: are they really the same disease? A 13-institution study from the US Extrahepatic Biliary Malignancy Consortium and the Central Pancreas Consortium. J Am Coll Surg. 2017;224(4):406–13.
DeOliveira ML, Cunningham SC, Cameron JL, et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg. 2007;245(5):755–62.
Riall TS, Cameron JL, Lillemoe KD, et al. Resected periampullary adenocarcinoma: 5-year survivors and their 6- to 10-year follow-up. Surgery. 2006;140(5):764–72.
Hatzaras I, George N, Muscarella P, Melvin WS, Ellison EC, Bloomston M. Predictors of survival in periampullary cancers following pancreaticoduodenectomy. Ann Surg Oncol. 2010;17(4):991–97.
Cameron JL, He J. Two thousand consecutive pancreaticoduodenectomies. J Am Coll Surg. 2015;220(4):530–36.
Konishi M, Iwasaki M, Ochiai A, Hasebe T, Ojima H, Yanagisawa A. Clinical impact of intraoperative histological examination of the ductal resection margin in extrahepatic cholangiocarcinoma. Br J Surg. 2010;97(9):1363–8.
Kiriyama M, Ebata T, Aoba T, et al. Prognostic impact of lymph node metastasis in distal cholangiocarcinoma. Br J Surg. 2015;102(4):399–406.
Murakami Y, Uemura K, Hayashidani Y, et al. Prognostic significance of lymph node metastasis and surgical margin status for distal cholangiocarcinoma. J Surg Oncol. 2007;95(3):207–12.
Squires MH, 3rd, Kooby DA, Poultsides GA, et al. Effect of perioperative transfusion on recurrence and survival after gastric cancer resection: a 7-institution analysis of 765 patients from the US Gastric Cancer Collaborative. J Am Coll Surg. 2015;221(3):767–77.
Postlewait LM, Squires MH, Kooby DA,Weber SM, Scoggins CR, Cardona K, Cho CS, Martin RCG, Winslow ER, Maithel SK. The relationship of blood transfusion with peri-operative and long-term outcomes after major hepatectomy for metastatic colorectal cancer: a multi-institutional study of 456 patients. HPB. 2015;1–8.
Kneuertz PJ, Patel SH, Chu CK, et al. Effects of perioperative red blood cell transfusion on disease recurrence and survival after pancreaticoduodenectomy for ductal adenocarcinoma. Ann Surg Oncol. 2011;18(5):1327–34.
Poorman CE, Postlewait LM, Ethun CG, et al. Blood transfusion and survival for resected adrenocortical carcinoma: a study from the United States Adrenocortical Carcinoma Group. Am Surg. 2017;83(7):761–68.
Sutton JM, Kooby DA, Wilson GC, et al. Perioperative blood transfusion is associated with decreased survival in patients undergoing pancreaticoduodenectomy for pancreatic adenocarcinoma: a multi-institutional study. J Gastrointest Surg. 2014;18(9):1575–87.
Gierth M, Aziz A, Fritsche HM, et al. The effect of intra- and postoperative allogenic blood transfusion on patients’ survival undergoing radical cystectomy for urothelial carcinoma of the bladder. World J Urol. 2014;32(6):1447–53.
Amato A, Pescatori M. Perioperative blood transfusions for the recurrence of colorectal cancer. Cochrane Database Syst Rev. 2006;(1):CD005033.
Keller SM, Groshen S, Kaiser LR. Blood transfusion and lung cancer recurrence. Ann Thorac Surg. 1989;48(5):746–47.
Manyonda IT, Shaw DE, Foulkes A, Osborn DE. Renal cell carcinoma: blood transfusion and survival. Br Med J (Clin Res Ed). 1986;293(6546):537–38.
Linder BJ, Thompson RH, Leibovich BC, et al. The impact of perioperative blood transfusion on survival after nephrectomy for non-metastatic renal cell carcinoma (RCC). BJU Int. 2014;114(3):368–74.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–74.
Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13.
Wang T, Luo L, Huang H, et al. Perioperative blood transfusion is associated with worse clinical outcomes in resected lung cancer. Ann Thorac Surg. 2014;97(5):1827–37.
Perisanidis C, Dettke M, Papadogeorgakis N, et al. Transfusion of allogenic leukocyte-depleted packed red blood cells is associated with postoperative morbidity in patients undergoing oral and oropharyngeal cancer surgery. Oral Oncol. 2012;48(4):372–78.
Miki C, Hiro J, Ojima E, Inoue Y, Mohri Y, Kusunoki M. Perioperative allogeneic blood transfusion, the related cytokine response and long-term survival after potentially curative resection of colorectal cancer. Clin Oncol (R Coll Radiol). 2006;18(1):60–66.
Hofer SO, Shrayer D, Reichner JS, Hoekstra HJ, Wanebo HJ. Wound-induced tumor progression: a probable role in recurrence after tumor resection. Arch Surg. 1998;133(4):383–89.
Hofer SO, Molema G, Hermens RA, Wanebo HJ, Reichner JS, Hoekstra HJ. The effect of surgical wounding on tumour development. Eur J Surg Oncol. 1999;25(3):231–43.
Pandey P, Chaudhary R, Aggarwal A, Kumar R, Khetan D, Verma A. Transfusion-associated immunomodulation: Quantitative changes in cytokines as a measure of immune responsiveness after one time blood transfusion in neurosurgery patients. Asian J Transfus Sci. 2010;4(2):78–85.
Innerhofer P, Tilz G, Fuchs D, et al. Immunologic changes after transfusion of autologous or allogeneic buffy coat-poor versus WBC-reduced blood transfusions in patients undergoing arthroplasty. II. Activation of T cells, macrophages, and cell-mediated lympholysis. Transfusion. 2000;40(7):821–27.
Hebert PC, Wells G, Blajchman MA, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–17.
Gani F, Cerullo M, Ejaz A, et al. Implementation of a blood management program at a tertiary care hospital: effect on transfusion practices and clinical outcomes among patients undergoing surgery. Ann Surg. 2017.
Shehata N, Whitlock R, Fergusson DA, et al. Transfusion Requirements in Cardiac Surgery III (TRICS III): study design of a randomized controlled trial. J Cardiothorac Vasc Anesth. 2018;32(1):121–29.
Jarnagin WR, Gonen M, Maithel SK, et al. A prospective randomized trial of acute normovolemic hemodilution compared to standard intraoperative management in patients undergoing major hepatic resection. Ann Surg. 2008;248(3):360–69.
Hallet J, Hanif A, Callum J, et al. The impact of perioperative iron on the use of red blood cell transfusions in gastrointestinal surgery: a systematic review and meta-analysis. Transfus Med Rev. 2014;28(4):205–11.
Acknowledgment
Funding provided in part by the Katz Foundation. Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454 and TL1TR000456. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lopez-Aguiar, A.G., Ethun, C.G., Pawlik, T.M. et al. Association of Perioperative Transfusion with Recurrence and Survival After Resection of Distal Cholangiocarcinoma: A 10-Institution Study from the US Extrahepatic Biliary Malignancy Consortium. Ann Surg Oncol 26, 1814–1823 (2019). https://doi.org/10.1245/s10434-019-07306-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1245/s10434-019-07306-x